ISSN : 2573-0320
Journal of Transmitted Diseases and Immunity
Sensitivity and Specificity of Clear View Malaria Dual Rapid TestÃÆââ¬Å¡Ãâî among Children Attending Hospital in Sengerema District, North-Western Tanzania
1Division of Livestock and Human Diseases Vector Control, Tropical Pesticides Research Institute, Arusha, Tanzania
2Department of Medical Parasitology and Entomology, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
- *Corresponding Author:
- Kweka EJ
Division of Livestock and Human Diseases Vector Control
Tropical Pesticides Research Institute
PO Box 3024, Arusha, Tanzania
Tel: +255 754 368748
E-mail: kwekae@tpri.or.tz
Received Date: August 24, 2017; Accepted Date: September 11, 2017; Published Date: September 21, 2017
Citation: Kweka EJ, Mazigo HD (2017) Sensitivity and Specificity of Clear View Malaria Dual Rapid Test® among Children Attending Hospital in Sengerema District, North-Western Tanzania. J Transm Dis Immun Vol.1 No.2:14
Abstract
Malaria cases management and treatment need to have accurate and reliable diagnostic tools. Thus the present study evaluated the sensitivity and specificity of the test against expert microscopy in febrile under-five children in Sengerema District, north-western Tanzania. A finger prick blood sample was obtained from each child, and thin and thick blood smears were prepared, stained with 10% Giemsa and examined under the light microscope and ClearView Malaria Dual rapid test. A total of 232 children were included in the study. Of these, 29 (12.50%, 95%CI, 8.20-16.8) children had a positive malaria microscopy and 44 (18.97%, 95%CI, 13.9-23.9) were positive in the rapid diagnostic test. The sensitivity of the rapid diagnostic test increased with increasing P. falciparum geometric mean parasite density from 46.43% (95%CI, 39.72-53.1) at <200 parasites/μL to 93.75% (95%CI, 90.50-97.01) at ≥ 200 parasites/μL. ClearView Malaria Dual rapid test is highly specific and its sensitivity increases with increase in parasite density. The test could be used in holoendemic areas but its use in areas with low malaria transmission needs further evaluation.
Keywords
Malaria; RDTs; Fever; Sensitivity; Specificity; Children under-fives; Tanzania
Abbreviations
ACTs: Artemisinin-based Combination Therapies; HRP-II: Histidine- Rich Protein-II; pLDH: Plasmodium Lactate Dehydrogenase; RDT: Rapid Diagnostic Test; WHO: World Health Organization
Background
The key to effective management of malaria cases is prompt, accurate diagnosis and malaria case management using effective antimalarial drug. For parasitological diagnosis, currently the WHO recommends the use of microscopy and/or rapid diagnostic tests (RDTs) for all patients with suspected malaria before initiation of treatment [1-4]. However, these recommendations have invited vigorous debate [3,5]. Despite several challenges faced by the use of microscopy, the technique remains as a gold standard for parasitological diagnosis of malaria in different endemicity settings [6]. To overcome these challenges and in areas where microscopy is usually not available; the WHO recommends the use of RDTs [5,7,8]. Malaria RDTs are immunochromatographic tests which targets specific single or multiple antigens of Plasmodium species [8,9]. At present, more than 200 different products of RDTs have been developed and over 60 brands are used for malaria diagnosis in different malaria endemic countries [10]. In Tanzania, ParaHIT-f, ParaCheck-Pf and SD Bioline are currently been used for malaria diagnosis (National Malaria Control Programme personal Communication). In fact, SD Bioline has been recommended by the National Malaria Control Programme for use in all public health facilities [11-15]. The use of malaria RDTs have resulted into the decrease of misdiagnosis and use of ACTs among febrile patients in malaria endemic areas [7,12].
One of the challenges facing the use of RDTs for malaria diagnosis is their low sensitivity in malaria endemic areas. In the holoendemic areas of north-western Tanzania, the sensitivity and specificity of ParaHIT-f was 29.8%. There was a strong association between parasitaemia of ≥ 1000 parasites/μL and being positive for ParaHIT-f diagnostic test [16]. When the same test was subjected to an area characterized by low malaria transmission, the sensitivity was 10.7% [15]. Sensitivity of ParaHIT-f increased with increasing P. falciparum mean parasite density from 5.8% at <100 parasites/μL to 20.5% at ≥ 100 parasites/μL [15].
Similarly, in the same country, the sensitivity and specificity of the ParaCheck-Pf have been reported by several previous studies to be above 90% and specificity of 95%-99% [11-13]. Conversely, SD Bioline although recommended for use in public health facilities in Tanzania, its sensitivity remains unknown or still under study, but in Malawi, the test had a sensitivity of 97% and specificity of 39% [17]. The second challenge facing RDTs is the declining of malaria transmission and malaria parasitaemia in different countries previously described as hyperendemic/holoendemic area [1,18]. The WHO cut-off point for measuring sensitivity of malaria RDT at low parasites density is ≥ 200 parasites/μL [1,18]. However, in areas with low malaria transmission, the proportional of individuals with low malaria parasites density (<200 parasites/μL) is higher as compared to areas with stable malaria transmission [19]. Therefore, using the WHO criteria for selecting RDTTs means that majority of the symptomatic malaria cases will be missed by the test in areas with low transmission [18,20,21]. Thus, if only RDTs will continue to be used for parasitological malaria diagnosis, there is a need to develop new tests with high sensitivity to detect malaria parasites below the WHO recommendations. One such test is the ClearView malaria Dual RDTs, designed to test in vitro the presence of P. falciparum, P. vivax, P. malariae and P. ovale antigens in whole blood. The test detects two types of parasite antigens: histidine-rich protein- II (HRP-II) and Plasmodium lactate dehydrogenase (pLDH) [22]. The detection of pLDH antigen by the test indicates the presence of viable parasites [22]. To our knowledge, two studies have evaluated the sensitivity and specificity of ClearView malaria dual RDTs in malaria non-endemic setting [21,22]. One of the studies reported an overall sensitivity of 93.2%, with that of P. falciparum recorded at 98.6% and specificity of 100% [22]. For P. falciparum, the sensitivity increased to 100% at parasites densities >100 parasites/μL [22]. ClearView Malaria Dual test have never been evaluated in malaria endemic settings in Tanzania, thus before this test can be used for malaria diagnosis at primary health facilities, it is important to evaluate its sensitivity and specificity. The objective of this study was to evaluate the sensitivity and specificity of the new brand of malaria RDT, ClearView malaria Dual against expert microscopy, the gold standard.
Methods
Study sites, design and population
The study was conducted at Sengerema Designed District Hospital, in Sengerema district, in Mwanza region, Northern west Tanzania. The study area has been previously described elsewhere [23]. Briefly, the area is located at 1140 m above sea level with the inland areas covered by seasonal rivers. The area is characterized by a tropical climate with average annual temperature of 26.5°C, and experiences short rain season between August and December and long rainy seasons between February and May. However, for the last year, the area received heavy rainfall from September to December which somehow increased malaria transmission in the area during the study period.
This was a cross-sectional study conducted between November- December 2011 among 232 febrile (body temperature ≥ 37.5°C) children under-fives. The inclusion criteria for the study participants were: (i) children who sought treatment at the hospital with complaint of fever (ii) children aged between 2 months and 60 months (iii) parents/guardians consent for their children to participate in the study (iv) no history of treatment with anti-malarial drugs in the past two weeks.
Data collections
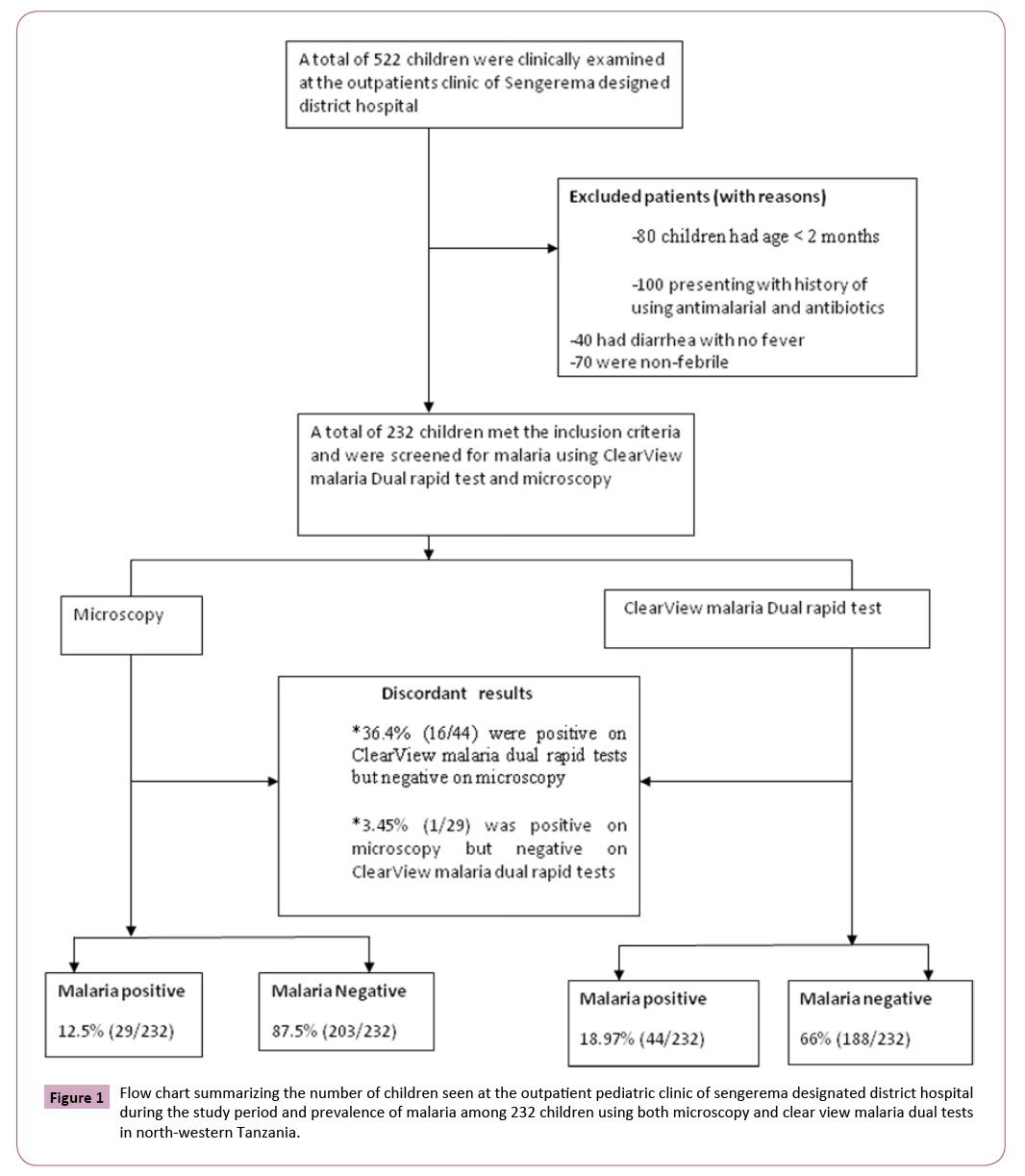
The under-five children were consequently recruited into the study until the sample size of 232 was reached (Figure 1). A convenient sampling technique was used and all children underfives meeting the inclusions criteria were recruited into the study. A qualified clinician used a pre-tested Kiswahili translated questionnaire to collect patient’s demographic information. The clinician conducted a physical clinical examination on all patients. The axillary body temperature was measured using a digital clinical thermometer. Fever was defined as body temperature ≥ 37.5°C [24].
Microscopic diagnosis of malaria
A finger prick blood sample was obtained and one thin and two thick blood smears were prepared and stained with 10% Giemsa. Giemsa stained slides were examined by two independent experienced microscopist at the National Institute for Medical Research, Mwanza, Tanzania. The process was based on a rigorous quality control system. All the microscopists were blinded to the RDT results. A second microscopist re-examined all the stained blood smears and any disagreement between the first and second microscopist was resolved by a third microscopist. The microscopist identified malaria parasite stages and speciate the malaria parasites. The Giemsa stained smears were examined at 100× objectives under oil immersion and a slide with the presence of trophozoites or any other asexual form of Plasmodium species was declared as positive. Slides were declared as negative if no asexual parasites were found after examining 100× objectives. Parasitaemia was determined according to the number of parasites per 200 white blood cells (WBC), assuming a total WBC count of 8,000/μL [24].
The diagnosis and management of malaria was based on initial readings of one of the thick blood smears at the hospital. Management of malaria was done in accordance with the Tanzania Malaria Treatment Guidelines.
ClearView test platform
ClearView test captures monoclonal antibodies (anti HRP2 and pLDH) which are immobilized on the nitrocellulose strip. The RBCs are lysed releasing plasmodium specific antigens which bind selectively to these antibodies as the blood is wicked up the strip. The signal reagent is coated with specific antibodies which bind with antibody-antigen complexes so formed producing a red line. The presence of an upper red line (the procedural control line) demonstrates the tests have been performed correctly. According to manufacturers, ClearView malaria Dual (Inverness Medical Group of companies; Plot No. 266, Sector 6, I.M.T., Manesar, Gurgaon-122050, Haryana India) detects in vitro the presence of P. falciparum, P. vivax, P. malariae and P. ovale. Handling, test performance and storage of the test kits was done according to manufacturer’s instructions.
The test was performed according to manufacturer instructions. Briefly, an approximate of 25 μL of the finger prick blood samples was collected using the provided disposable pipettes. Then, five drops of the reactions buffer was added into the large well and readings of the reactions were performed after 20 minutes. Results were interpreted as positive if two red colored lines were observed (the middle test (T) line and top control (C) for P. falciparum malaria positive. For non P. falciparum malaria positive, two red lines appeared (the bottom test (T) line and top control (C)). The presence of only the control (C) line with no either test line, was considered negative.
Data analysis
Data were entered and verified using MS-Access, and analyzed using Stata version 11 software (Stata Corp, College Station, TX, USA). Specificity, sensitivity and predictive values of ClearView Malaria dual rapid tests and their respective 95% confidence intervals were estimated using expert microscopy (gold standard). ClearView Malaria dual rapid tests were also tested across different parasite densities. Parasitaemia was categorized as either low (<200 parasites/μL) or high (≥ 200 parasites/μL).
Ethical consideration
The study received ethical clearance from the joint Institutional Ethical Review Board of Weill-Bugando University College of Health Sciences/Bugando Medical Center, Mwanza, Tanzania. In addition, the study received permission from the District Medical Officer of Sengerema district and Sengerema Designated District Hospital management. A written consent was thought from guardians/parents of children under five before recruited into the study.
Results
A total of 232 under five children participated in the present study, of these 129/232 (55.60%, 95%CI, 49.21- 61.99) were females and the remaining were males. The median age of the enrolled children was 16.5 months (range-minimum-maximum: 1-60 months).
Based on the microscopic diagnosis of malaria, of the 232 children, only 12.50% (29/232, 95%CI, 8.24-16.76) had positive slide readings. All the Plasmodium positive children were positive for P. falciparum infection. The geometric mean of parasites density was 447.14 parasites/μL (95%CI, 214.98-929.99). Of the children with P. falciparum slides positives, 57.69% (15/26, 95%CI, 38.7-76.69) had 1- 1000 parasites/μL and 46.15% 12/26, 95%CI, 26.99-65.31) had ≥ 1001 parasites/μL, respectively.
The ClearView malaria Dual rapid test detected 44 (18.97%, 95%CI, 13.90-23.90) out of 232 febrile children underfives. All 18.97% (95%CI, 13.90-23.90) malaria cases had P. falciparum malaria. The prevalence of malaria detected by ClearView malaria Dual rapid test was higher than what was detected by expert microscopy (18.97% versus 12.50%) and the observed difference was statistically significant (χ2=129.8149, P=0.0001).
A total of 17 febrile children had discordant results. Most frequent were positive ClearView malaria rapid tests results and negative microscopic slide findings. In 16 (36.36%, 95%CI, 22.15-50.57) of the 44 febrile children detected as P. falciparum malaria positive by ClearView Malaria Dual rapid test, were microscopic slide negative. Only one febrile child had negative ClearView malaria dual rapid test but with positive microscopic (parasite density of 1640 parasites/μL).
The overall sensitivity and specificity of ClearView malaria Dual rapid test was 96.55% (44/232, 95%CI, 94.10-98.86) for diagnosis of P. falciparum and specificity of 92.12% (95%CI, 88.62-95.60). The positive and negative predictive values were 63.64% and 99.47%, respectively.
Based on parasitaemia, the sensitivity of the test was observed to increase from 46.43% (95%CI, 39.72-53.10) (specificity of 99.5%) at parasite density of <200 parasites/μL to 93.75% (95%CI, 90.50- 97.01) (specificity 100%) at parasite density of ≥ 200 parasites/ μL (Table 1).
Table 1 Overall sensitivity, specificity, positive and negative predictive values (PPV, NPV) of ClearView malaria dual rapid test and stratified results according to parasite density.
| Parasitaemia (Parasites/µL) | Sensitivity (95%CI) |
Specificity (95%CI) | PPV (95%CI) | NPV (95%CI) |
|---|---|---|---|---|
| <200 | 46.43 (39.72-53.1) | 99.50 (98.54-100.00) | 100 | 92.12 (89.12-95.83 ) |
| = 200 | 93.75 (90.50-97.01) | 100 | - | - |
| Overall | 96.55 (94.10- 98.86) |
92.12 (88.62-95.60) | 63.64 (49.43-77.85) | 99.47 (98.44-1.00) |
Discussion
This study have found that, parasitological diagnosis of malaria before treatment decision is highly important as recommended by WHO especially at this time when malaria is reported to decline in most of the endemic areas [12,25-28]. The use of light microscopy or RDTs offers an inexpensive and practical means of improving malaria diagnosis and treatment in areas of low transmission [10,29]. An important aspect on the selection of the type of malaria RDTs brands to be used in malaria endemic areas depends on its sensitivity and specificity. In this cross-sectional study, the sensitivity and specificity of the ClearView Malaria Dual rapid test® was evaluated against expert microscopy as gold standard among febrile children under five. The overall sensitivity of the tests was 97% for the detection of only P. falciparum malaria. In Tanzania, over 95% of the malaria cases are due to P. falciparum [24]. The sensitivity of the test was observed to increase considerably with parasite density, from 46.43% at parasite density of <200 parasites/μL to 94% at parasite density of >200 parasites/μL. Meaning that, in areas with low malaria transmission with majority of the population having >200 parasites/μL, the tests will diagnose majority of the patients as false-negative. Thus the use of this test kit will have limited use in areas with low malaria transmission and the test will achieve better performance in areas with high malaria transmission.
The findings observed in the present study were also reported by similar other studies in malaria non-endemic areas [9,21]. The overall sensitivity of diagnosing P. falciparum for ClearView Malaria dual rapid tests were reported to range between 98- 99% reaching 100% for samples having more than 100 parasites/ μL [9]. For the level of parasites to be detected by RDTs, the WHO recommends a sensitivity of 95% at parasitaemia of 100 parasites/μL as a target for RDT performance [10]. However, in the present study, the sensitivity of ClearView Malaria Dual rapid tests was observed to be below the WHO recommendation (46.43% at parasite density of <200 parasites/μL). This was also inconsistent with the findings of another similar study which reported a sensitivity of 100% at parasite density of >100 parasites/μL of the same malaria RDTs [9]. Similarly, when comparing the sensitivity of other malaria RDTs at parasites density <100 parasites/μL, for example ParaCheck-Pf (PfHRP-2) and Optimal-IT test, their sensitivity were <50% [11,12,30]. In fact, parasite density of <200 asexual parasites/μL was reported to be associated with high risk of false negative in RDTs [12]. In addition, the observed declining of malaria transmission means that the largest proportional of the population will be living in areas with low malaria transmission, characterized by low malaria parasites density (<200 parasites/μL), thus malaria RDTs which have low sensitivity at parasite density of <200 parasites/ μL will have limited use [18,20,24]. Conversely, the WHO have confirmed that at parasites <200 parasites/μL, detection rates of most of RDTs decreases substantially. Thus for selection of RDTs to be used in malaria endemic areas, the detection score should be at least 50% for P. falciparum at parasites counts of 200 parasites/μL [31].
The overall specificity of the tests for diagnosing P. falciparum malaria was >90% and based on the level of parasitaemia, the specificity was excellent, with no significant difference at parasite density of <200 or ≥ 200 parasites/μL. Only one false-negative result was observed with parasite density of 1640 parasites/μL. Similar observation has been reported by previous study but this study did not observe any false-negative result [9].
In general, evaluation of the present test in hospital setting in malaria endemic setting have shown that, its sensitivity rates and specificity are within the WHO recommendation at parasites density ≥ 200 parasites/μL. Similar to another report, the test was easier to perform and the bands lines (both control and test) were easily observed and interpreted by the laboratory technician [9].
Conclusion
In conclusion, the present study has shown that ClearView Malaria Dual rapid test is highly specific and its sensitivity tends to increase with increase in level of parasitaemia. Thus, the tests can be used for diagnosis of P. falciparum malaria in children living in holoendemic for malaria transmission. However, its use in low malaria transmission areas needs further evaluation.
Competing Interests
The authors declare that they have no any competing interest
Authors’ Contributions
EJK and HDM have contributed equally in designing, data analysis and drafting of the manuscript. EJK and HDM read and approved the last version of the manuscript for submission.
Funding
This study received no any financial aid but had utilized resources from ongoing projects.
Acknowledgements
We thank the parents/guardians/caregivers for allowing their children under-fives to participate in this study. We express our thanks to all the laboratory technicians and the clinicians. Authors appreciate funding given to this study from other projects at Catholic University of Health and Allied Sciences.
References
- Abeku TA, Kristan M, Jones C, Beard J, Mueller DH, et al. (2008) Determinants of the accuracy of rapid diagnostic tests in malaria case management: evidence from low and moderate transmission settings in the East African highlands. Malaria Journal 7: 202.
- Njama-Meya D, Clark TD, Nzarubara B, Staedke S, Kamya MR, et al. (2007) Treatment of malaria restricted to laboratory-confirmed cases: a prospective cohort study in Ugandan children. Malaria Journal 6: 7.
- Graz B, Willcox M, Szeless T, Rougemont A (2011) "Test and treat" or presumptive treatment for malaria in high transmission situations? A reflection on the latest WHO guidelines. Malaria Journal 10:136.
- WHO: Parasitological confirmation of malaria diagnosis: (2010) WHO technical consultation, Geneva, 6-8 October 2009. Geneva: World Health Organization.
- D'Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, et al. (2009) Time to move from presumptive malaria treatment to laboratory-confirmed diagnosis and treatment in African children with fever. PLoS Medicine 6:e252.
- Kahama-Maro J, D'Acremont V, Mtasiwa D, Genton B, Lengeler C (2011) Low quality of routine microscopy for malaria at different levels of the health system in Dar es Salaam. Malaria Journal10:332.
- Bastiaens GJ, Schaftenaar E, Ndaro A, Keuter M, Bousema T, et al.(2011) Malaria diagnostic testing and treatment practices in three different Plasmodium falciparum transmission settings in Tanzania: before and after a government policy change. Malaria Journal 10:76.
- WHO: World malaria report. (2010) Malaria diagnosis and treatment. Geneva: World Health Organization,pp:25-38.
- Houze S, Hubert V, Cohen D, Rivetz B, Le Bras J (2011) Evaluation of the Clearview(R) malaria pLDH malaria rapid diagnostic test in a non-endemic setting. Malaria Journal 10:284.
- WHO: Malaria Rapid Diagnostic Test Performance; Results of WHO product testing of malaria RDTs: round 2 (2009). Geneva: World Health Organisation; 2010.
- Mboera LE, Fanello CI, Malima RC, Talbert A, Fogliati P, et al.(2006) Comparison of the Paracheck-Pf test with microscopy, for the confirmation of Plasmodium falciparum malaria in Tanzania. Annals of Tropical Medicine and Parasitology 100:115-122.
- Ishengoma DS, Francis F, Mmbando BP, Lusingu JP, Magistrado P, et al.(2011) Accuracy of malaria rapid diagnostic tests in community studies and their impact on treatment of malaria in an area with declining malaria burden in north-eastern Tanzania. Malaria Journal 10:176.
- Kamugisha ML, Msangeni H, Beale E, Malecela EK, Akida J, et al. (2008) Paracheck Pf compared with microscopy for diagnosis of Plasmodium falciparum malaria among children in Tanga City, north-eastern Tanzania. Tanzania Journal of Health Research 10:14-19.
- Kamugisha E, Mazigo HD, Manyama M, Rambau P, Mirambo M, et al. (2009) Low sensitivity but high specificity of ParaHIT-F in diagnosing malaria among children attending outpatient department in Butimba District Hospital, Mwanza, Tanzania. Tanzan J Health Res 11:97-99.
- Kweka EJ, Lowassa A, Msangi S, Kimaro EE, Lyatuu EE, et al.(2011) Low sensitivity of ParaHIT-f rapid malaria test among patients with fever in rural health centers, Northern Tanzania. Journal of Infection in Developing Countries 5:204-208.
- Kamugisha E, Mazigo H, Manyama M, Rambau P, Mirambo M, et al. (2009) Low Sensitivity but High Specificity of ParaHIT-f in diagnosing malaria among children attending outpatient department in Butimba district hospital, Mwanza, Tanzania. Tanzan J Health Resl 11:97-99.
- Chinkhumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, et al (2010) Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malaria Journal 9:209.
- McMorrow ML, Aidoo M, Kachur SP (2011) Malaria rapid diagnostic tests in elimination settings--can they find the last parasite? Clin Microbiol Infect 17:1624-1631.
- Harris I, Sharrock WW, Bain LM, Gray KA, Bobogare A, et al. (2010) A large proportion of asymptomatic Plasmodium infections with low and sub-microscopic parasite densities in the low transmission setting of Temotu Province, Solomon Islands: challenges for malaria diagnostics in an elimination setting. Malaria Journal 9:254.
- WHO: Guidelines for the treatment of malaria. Geneva,World Health Organization2010.
- Marx A, Pewsner D, Egger M, Nuesch R, Bucher HC, et al. (2005) Meta-analysis: accuracy of rapid tests for malaria in travelers returning from endemic areas. Annals of Internal Medicine 142:836-846.
- Houze S, Hubert V, Cohen DP, Rivetz B, Le Bras J (2011) Evaluation of the Clearview(R) Malaria pLDH Malaria Rapid Diagnostic Test in a non-endemic setting. Malaria Journal 10:284.
- Mazigo HD, Waihenya R, Lwambo NJ, Mnyone LL, Mahande AM, et al. (2010) Co-infections with Plasmodium falciparum, Schistosoma mansoni and intestinal helminths among schoolchildren in endemic areas of northwestern Tanzania. Parasites & Vectors 3:44.
- Mazigo HD, Meza W, Ambrose EE, Kidenya BR, Kweka EJ (2011) Confirmed malaria cases among children under five with fever and history of fever in rural western Tanzania. BMC Research Notes 4:359.
- Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, et al. (2008) Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372:1545-1554.
- Okiro EA, Hay SI, Gikandi PW, Sharif SK, Noor AM, et al. (2007) The decline in paediatric malaria admissions on the coast of Kenya. Malaria Journal 6:151.
- O'Meara WP, Mangeni JN, Steketee R, Greenwood B (2010) Changes in the burden of malaria in sub-Saharan Africa. The Lancet Infectious Diseases 10:545-555.
- Mmbando BP, Vestergaard LS, Kitua AY, Lemnge MM, Theander TG, et al. (2010) A progressive declining in the burden of malaria in north-eastern Tanzania. Malaria Journal 9:216.
- Reyburn H, Ruanda J, Mwerinde O, Drakeley C (2006) The contribution of microscopy to targeting antimalarial treatment in a low transmission area of Tanzania. Malaria Journal 5:4.
- Hendriksen IC, Mtove G, Pedro AJ, Gomes E, Silamut K, et al. (2011) Evaluation of a PfHRP2 and a pLDH-based rapid diagnostic test for the diagnosis of severe malaria in 2 populations of African children. Clin Infect Dis 52:1100-1107.
- Brentlinger PE, Behrens CB, Kublin JG (2007) Challenges in the prevention, diagnosis, and treatment of malaria in human immunodeficiency virus infected adults in sub-Saharan Africa. Archives of Internal Medicine 167:1827-1836.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences