ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Removal of Cadmium(II) Ions from Waste Water by Adsorption onto the Powder of Lebanese Anacyclus nigllifolius Boiss: A Comparative Study
Mohammad R1, Akram H1*, Rim H1, Ziad D1, Mostafa H1, Aqeel M2, Mounir K1 and Wassef W1
1Doctoral School of Science and Technology, Research Platform for Environmental Science (PRASE), Lebanese University, Lebanon
2Department of Biology, College of Science, Al Mustansiriya University, Lebanon
- *Corresponding Author:
- Akram H
Doctoral School of Science and Technology
Research Platform for Environmental
Science (PRASE), Lebanese University
Lebanon
Tel: 0096171905768
E-mail: akram.hijazi@ul.edu.lb
Received date: May 11, 2017; Accepted date: May 30, 2017; Published date: June 03, 2017
Citation: Mohammad R, Akram H, Rim H, et al. Removal of Cadmium(II) Ions from Waste Water by Adsorption onto the Powder of Lebanese Anacyclus nigllifolius Boiss: A Comparative Study. Am J Phytomedicine Clin Ther. 2017, 5:1. doi:10.21767/2321-2748.100324
Abstract
In this study, Anacyclus nigllifolius Boiss was adopted as a natural adsorbent to eliminate the cadmium from aqueous solutions. The adsorption was found to be dependent on initial metal ion concentration, pH of the solution, contact time and adsorbent dose. Characterization of the plant by granulometry has shown heterogeneity of size varying from 5.12 to 517.2 µm. The FT-IR spectrum showed a chemical modification of the functional groups due to the adsorption of cadmium. The adsorption of cadmium on the plant reaches 92% at ambient temperature. Experimental data of adsorption were well explained by Sips model with a maximal capacity of adsorption of 69.14 mg/g. Kinetics study showed that the retention of cadmium was well fitted by the pseudo-first-order model. The results of this study reveal that the Lebanese plant is a very effective and environmentally friendly adsorbent, with low cost for Cd(II) removal.
Keywords
Adsorption; Anacyclus nigllifolius Boiss; Cadmium; Isotherm; Kinetic
Introduction
The problem of pollution has increased with the growth and development of the industrial sector. This sector provides benefits to humans; it causes long-term environmental problems such as toxicity and pollution discharge their pollutants, for example: toxic gases, nuclear waste [1]. Heavy metals are very abundant present at high concentration in liquid industrial waste and are often deposited directly in the environment without any pre-treatment in the environment. Among these metals, Cadmium is considered one of the most dangerous heavy metals for human health and the environment [2]. That is why it is important to eliminate this element from water to protect the public health. Different techniques have been used for the decontamination of heavy metals. Within these techniques we can cite Reverse Osmosis, in which heavy metals are separated by a semi-permeable membrane at a greater pressure than that of osmotic pressure, however, this technique is expensive. Ionexchange technique has been broadly used to remove heavy metals from wastewater [3]. In this process, metal ions from dilute solutions are exchanged with ions held by electrostatic force on the exchange resin [3], however partial removal of certain ions as well as its high cost limited the use of this technique. In the Electro-dialysis technique, the ionic components, heavy metals in our case, are separated through semi-permeable ion selective membranes. The disadvantage of this method is the formation of metal hydroxides blocking the membrane. To remove heavy metals from inorganic effluents, the most widely used technique is chemical precipitation of metals by addition of coagulants as lime and other organic polymers [4,5]. The large amount of sludge containing toxic compounds produced during the process is the main disadvantage [4]. All of these methods have disadvantages such as incomplete metal removal, high reagent and energy requirements, generation of toxic sludge or other waste products [6]. However, adsorption is still one of the best and preferable techniques because it leads to eliminate the pollutants without ruining the quality of the water, which makes this approach very interesting mostly if the adsorbent is at a low price and available locally. This study systematically investigates and compares its performance as effective adsorbent to remove Cd(II) ions, as well as the factors influencing sorption, including contact time of interaction, PH, dose of the adsorbent and initial concentration. Adsorption isotherm and kinetic parameters have been estimated from experimental results. These findings would develop our essential understanding about to Cd(II) adsorption mechanisms to better design the water treatment process applications. The experimental results can help us to determine the optimum conditions for environmental applications.
Materials and Methods
Adsorbent preparation
Anacyclus nigllifolius Boiss utilized in this study was collected from South Lebanon in spring 2016. The collected plants were carefully washed with ultra-pure water to remove impurities, and then dried at room temperature for one week then in an oven at 60°C for 2 days. After drying, the dried plant was grinded using a grinding mill (MF 10 basic Microfine grinder drive) to get a powder that should be stocked in plastics sacs [7].
Characterization of adsorbent
Granulometric analysis: The granulometry is used to measure the distribution and the size variation of the particles and to know the statistical frequency of the different granulometric classes constituting this formation. This technique is based on the principle of diffraction and diffusion of a laser beam, and it is used for particles ranging from a few hundred nanometers to several millimeters.
FT-IR analysis: FT-IR analysis spectrometer was applied to determine the surface functional groups interfering in the adsorption of Cadmium by Anacyclus niglifollius Boiss /metal ions, where the spectra were recorded in the range of 4000 to 400 cm-1.
Batch adsorption studies
Stock solution of 200 mg/L of Cd(II) was prepared by dissolving Cd(II) in ultra-pure water. Other solutions with different concentrations were then prepared by dilution of the stock solution. Batch experiments were carried out using Erlenmeyer flasks (50 ml) where 50 ml of solution of Cd(II) were mixed with 100 mg of Anacyclus niglifollius Boiss at room temperature. The pH of solutions was adjusted by adding dilute solutions of HCl (0.1 M) and NaOH (0.1 M). Solutions are then stirred with a magnetic stirrer for one hour, whereas, for studies of the effect of the contact time, stirring time was different. After stirring, the adsorbent was filtered with filter-paper by Buchner filtration followed by a microfiltration using a micro filter of 0.45 μm. The filtrate was collected and analyzed by atomic adsorption spectrometer (AAS) to determinate the concentration of Cd(II) in the solutions. Metal uptake q (mg/g) and the percentage of removal (%) were calculated using the general equations 1 and 2 [8].
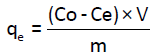 (1)
(1)
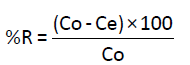 (2)
(2)
Where C0 and Ce are the initial and final metal concentrations in solution (mg/g) respectively, V0 and Vf are the initial and final solution volumes (l), respectively and m is the mass of the adsorbent used (mg).
Different conditions were been studied including Anacyclus niglifollius Boiss dose (25-300 mg), initial Cd(II) concentration (10-200 mg/l), contact time (5-120 min) and pH (2-10) [9].
Equilibrium studies (effect of initial concentration): 100 mg of Anacyclus niglifollius Boiss was added to glass flasks containing 50 ml of Cd (II) solution. The cadmium concentration was varied in the range of 10–200 mg/l, for a contact time of 1 h. The pH is adjusted at 4.
Kinetic studies (the effect of contact time under initial adsorbate concentration): The adsorption kinetic measurements for cadmium were performed in a batch system using a 100 mL glass bottles. In addition to 100 mg adsorbent, 50 mL of the cadmium solution were added to each flask, mixed and allowed to contact at different contact time durations between 5 to 120 min. The solutions were stirred using a magnetic stirrer with adopting controlled temperature conditions (20°C). Samples were collected at the predetermined time intervals (in regular periods of time).
Effect of pH: To study the effect of pH, batch experiments were prepared by mixing 50 ml of cadmium solution with 100 mg of adsorbent for 1 h adjusting continuously the solution pH at several values varying from 2 to 10 and at room temperature.
Effect of adsorbent dose: To study the effect of adsorbent dose on removal of cadmium, different amounts of Anacyclus (25 mg to 300 mg) were taken and agitated with 50 mL of cadmium solution for one hour. The pH is adjusted at 4; the flasks were stirred at room temperature.
Adsorption isotherms: Equilibrium data, generally known as adsorption isotherms, are important in the basic design of adsorption systems and are critical in optimizing the use of adsorbents. To optimize the design of an adsorption system for removing Cadmium from solutions, it is essential to establish the most appropriate correlation for the equilibrium curves. In the present study, nonlinear methods were used to estimate the isotherm parameters of Langmuir, Freundlich and Sips models for a better understanding of the interactions between the plant and the cadmium adsorbent. Furthermore, the isotherm parameters were evaluated by non-linear regression analysis using OriginPro.
Langmuir isotherm: The Langmuir isotherm is based on the assumption that the adsorption occurs at specific homogeneous sites within the adsorbent and there is no significant interaction among adsorbed species. The adsorbent is saturated after the formation of one layer of the adsorbed molecules on the adsorbent surface [10].
The Langmuir isotherm is represented by Eq. (3):
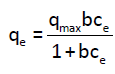 (3)
(3)
With qe: Amount of cadmium adsorbed at equilibrium (mg/g), qmax: Maximum adsorption capacity in (mg/g), b: Langmuir adsorption constant related to the energy of adsorption in L/mg and, Ce: Equilibrium concentration (mg/L).
Freundlich isotherm: The empirical Freundlich model [11] describes the adsorption on a heterogeneous surface and may be formulated through the Eq. (4):
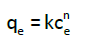 (4)
(4)
Sips isotherm: The Sips isotherm is a three-parameter isotherm that derived by combination of the Langmuir and Freundlich isotherms. The Sips equation [12] is similar in the form to the Freundlich equation, but it has a finite limit when the concentration is sufficiently high, as given by Eq. (5):
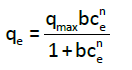 (5)
(5)
where qmax is the Sips maximum adsorption capacity (mg/g), b is the Sips equilibrium constant (L/mg)n, and n is the Sips model exponent. The linear regression was frequently used to determine the most fitted isotherm; the non-linear method offered a better way to predict the equilibrium isotherm parameters. Here, the determination of adsorption parameters (isotherm constants) was performed by using non-linear regression (Table 1).
kinetics studies: For the interpretation of the kinetic data, two pseudo-first-order (Eq.6) and second-order (Eq.7) kinetic model were used. The non-linearized form of the pseudo-first order equation is generally expressed as:
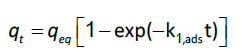 (6)
(6)
And the pseudo-second order kinetic model is represented by the following equation:
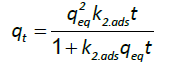 (7)
(7)
Where:
qt: concentration of the adsorbate in the adsorbent at time t (mg/g adsorbent),
qeq: equilibrium concentration of the adsorbate in solid phase (mg/g),
k1,ads :adsorption constant of the pseudo-first-order model (min-1),
k2,ads : adsorption constant of the pseudo-second-order model.
Results and Discussion
Adsorbent characterization
Granulometric analysis: This Figure 1 shows the size distribution of the powder of Anacyclus nigllifolius Boiss. This powder of 1 mm ground, presents heterogeneity of size with an average size of 114.4 μm. The particle size varies between 5.12 and 517.2 μm. In general, a smaller size increases the adsorption rate because it facilitates the arrival of the pollutant to the internal sites of the plant [13-15].
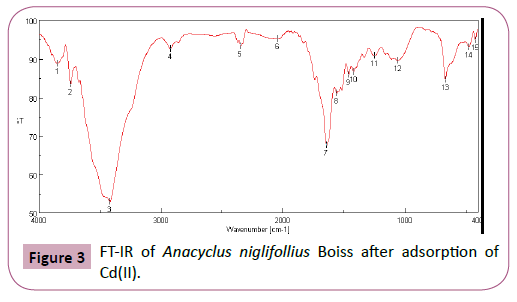
FT-IR analysis: The surface chemical analysis and surface functional groups studies for the plant Anacyclus nigllifollius Boiss were performed by using FTIR. The FTIR spectra were recorded in the 4000-400 cm-1 region before and after the adsorption of the Cd(II) ions. Figures 2 and 3 show the FT-IR spectrum before and after adsorption. The most intense peak at 3100 ~ 3620 cm-1 corresponds to vibrations of amines and hydroxyl groups. The decrease in peak intensities of these groups up to 3400 cm-1 and the chemical modification correspond to the change of the environment due to the adsorption of Cd(II). The peak at 2920 cm-1 corresponds to the elongation vibrations of the C-H groups. The spectra also show peaks at 1745, 1610 and 1508 cm-1 which are attributed to the carboxyl groups and the elongation vibrations of C=C bonds and N-H bonds.
Adsorption of cadmium
Effect of initial concentration: In this section, we describe the behavior of the plant Anacyclus nigllifolius Boiss on the adsorption of cadmium by varying the initial concentration of this metal. The different metal concentrations used range from 10 to 200 mg/l. All samples were treated at room temperature and stirred using magnetic stirrers. The Figure 4 shows the variation of the quantity of the adsorbed pollutant (qe) as a function of the equilibrium concentration for cadmium. It is clear that the adsorption capacity increases with increasing of the metal concentration from 2.45 to 70.744 mg/g. We also observe a high adsorption of cadmium. Thus, an interaction between the metal ions and the absorbent takes place: between the active sites of the plant and the metal cadmium. The best fitting equation is based on the highest R2 and the lowest R2. Evaluating the parameters shown in Table 1, the combined Langmiur-Freundlich isotherm expression (Sips) provides the best fit and it is the most suitable model for characterizing the adsorption of the cadmium. This means that the adsorption of cadmium on Anacyclus possibly occurred according to the conditions assumed in the Sips model. In other words, the adsorption is a monolayer phenomenon and the adsorption sites are not identical to each other.
| Isotherm modeling parameters for Sips model | χ2 | 14.43784 |
| R2 | 0.9778 | |
| qmax | 69.1402mg/g | |
| b | 6.57×10-4 |
Table 1: Isotherm modeling parameters.
Effect of contact time: The study of the adsorption of cadmium on Anacyclus nigllifolius Boiss over time allows the determination of contact time. It’s the time which corresponds to the adsorption equilibrium. We studied the effect of time on the adsorption for a time varying from 5 to 120 min. As shown in Figure 5, the Anacyclus nigllifolius Boiss plant was able to remove 93% Cd (II) for 75 min. We observe that the percentage of elimination during the first 5 minutes is approximately 48.98%, and then this percentage increases to reach a value of 91.62% after 30 min of agitation. A small increase of 92.19% to 93.34% of the removal percentage is detected when the time increases from 30 min to 75 min. After 75 min, the percentage of elimination became constant over time. So we reach the equilibrium time, which means that the adsorption sites are saturated. Rapid adsorption during the initial stage is due to the high availability of sorption sites during this step. The value of the constant (k) and the cadmium correlation coefficients (R2) obtained using the pseudofirst- order and pseudo-second-order kinetics models are given in Table 2. The value of R2 Indicates that the pseudo-second order model is inappropriate for describing adsorption reactions of cadmium by Anacyclus nigllifolius Boiss whereas the pseudo-first order model presents the ideal model for the description of the adsorption of cadmium by the plant.
| Models | Parameters | Cadmium |
|---|---|---|
| Pseudo-first-order | qe(mg/g) R2 K1(min-1) χ2 |
18.4 0.991 0.121 0.911 |
| Pseudo-second-order | qe(mg/g) R2 K2(g/mg/min) χ2 |
12.56 0.841 0.007 0.845 |
Table 2: Parameters of kinetics study.
Effect of pH: The study of the adsorption of cadmium on Anacyclus nigllefolius Boiss is carried out for 2 hours at pH varying between 2 and 10 with the same concentration of Cadmium solutions. The best results are obtained at pH between 4 and 6, where the elimination percentage is between 88.9% and 91.09%. The percentage of elimination of cadmium by the plant Anacyclus niglifollius Boiss increases with the increase in pH to reach a maximum value of 91.09% at pH 6. The percentage of cadmium removal is minimal with a percentage of 64.22% when the pH is 2. We can therefore say from Figure 6 that the pH has a significant effect and influences the adsorption of cadmium. This indicates that at low pH (very acid medium), the ions hardly adsorb to the surface because it is already saturated by H+ ions. By increasing the pH, there will be an increase in the adsorption capacity since the competition between the H+ ions in solution and the Cd (II) metals decreases and consequently the Cd (II) metal ions can readily adsorb to the surface of plant. Moreover, for a very high pH, the Cd (II) ions precipitate in the form of Cd(OH)2 and consequently the adsorption capacity decreases.
Effect of adsorbent dose: The study of the effect of the plant dose on the adsorption capacity of Cd consisted of varying the dose of this plant (25 mg, 50 mg, 100 mg, 150 mg, 200 mg, 250 mg and 300 mg), while maintaining the initial concentration of cadmium. The results of Figures 6 and 7 shows that the increase in the dose of the plant Anacyclus nigllifollius Boiss influences the percentage of elimination of the adsorbed cadmium until reaching a maximum value of 90% for a dose of the plant greater than 100 mg. In other words, the more the mass of the plant increases, the more the cadmium is adsorbed, to a value corresponding to saturation. Any further addition of the plant does not cause any significant change in adsorption capacity. The increase in the adsorption capacity of cadmium with the increase in the dose of the adsorbent is attributed to the availability of a larger surface for adsorption and consequently the availability of the sites increases. This has the consequence of increase of interaction (molecule-site), and consequently better retention of cadmium. According to Table 3, and comparing the adsorption capacities of Anacyclus nigllifolius Boiss and other adsorbents, we can say that this plant has the higher capacity for the removal of Cd (II) from aqueous solutions. The difference in elimination of this metal is due to the properties of each adsorbent as the experimental conditions are the same for all these experiments.
| Differentadsorbents | qmax | References |
|---|---|---|
| AnacyclusnigllifolliusBoiss | 69.14 | Ourstudy |
| Algae | 80 | [13] |
| Bentonite | 9.27 | [14] |
| Activatedcarbon | 11.27 | [14] |
| Diatomite | 3.24 | [14] |
| Kaolin | 3.04 | [14] |
| Montmorillonite | 0.153 | [15] |
Table 3: Adsorption capacity of this study compared with various adsorbents as reported in literature.
Conclusion
The objective of our study is to study the ability of Anacyclus niglifollius Boiss to retain and adsorb cadmium from aqueous solutions. The main results are the Anacyclus niglifollius Boiss powder presents heterogeneity of size ranging from 5.12 to 517.2 μm. Adsorption of cadmium on the plant reaches 92% at room temperature.- The adsorption process of cadmium is represented by the Sips model and by the pseudo-first order model. In conclusion, this plant, at low cost and with no negative effects on the environment, has a great capacity to eliminate cadmium. This study should be continued and tested to remove the heavy metals and other organic pollutants from the water.
Acknowledgments
The authors are thankful to the Lebanese university for supporting this work
References
- WirtgenJ(2009) Facts on water resources.
- Jarup L, Berglund M, Elinder CG, Nordberg G (1998) Health effects of cadmium exposure, a review of the literature and a risk estimate.Sciand J Work EnvironHealth24:1-51.
- Kang KW, Le SY, Moon JU,Kim SH (2004)Competitive adsorption characteristics of Co, Ni, and Cr by IRN-77 cation exchange resin in synthesized wastewater. Chemosph56:141-147.
- Rao LN (2011) Removal of heavy metals by biosorption -an overall review.J Eng Res Stud.
- Wang NK, Vaccari LK, Li Y (2004) Chemical precipitation.Humana Press, New Jersey,Physicochem TreatProcess3: 141-198.
- Ozcan A, Ozcan AS, Tunali S, Akar T(2005) Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper(II) ions onto seeds of Capsicum annuum. JHazardMater124: 200-208.
- Sobh M, Moussawi MA, Rammal W, Hijazi A (2014) Removal of Lead (II) Ions from Waste Water by Using Lebanese Cymbopogon citratus (Lemon Grass) Stem as Adsorbent. American Journal of Phytomedicine and Clinical Therapeutics2: 1070-1080.
- AlAfy N, Hijazi A, Rammal H, Reda M (2013) Adsorption of Chromium (VI) from Aqueous Solutions by Lebanese Prunus avium Stems.American Journal of Environmental Engineering3: 179-186.
- Asnaoui H, Laaziri A, Khalis M (2015)Study of the kinetics and the adsorption isotherm of cadmium(II) from aqueous solution using green algae (Ulva lactuca) biomass. Water Science and Technology72:1505-1515.
- Gherbi N (2008)Experimental study and identification of the process of metal cations by natural materials.PhD Thesis in Process Engineering SciencesAlgeria.
- Freundlich H(1906) J Phys Chem 57: 385.
- Sips R(1948) J Chem Phys 16: 490.
- Al-othman ZA, Hashem A, Habila MA (2011) Kinetic, equilibrium and thermodynamic studies of cadmium (II) adsorption by modified agricultural wastes.Molecules16: 10443-10456.
- Ulmanu M, Maranon E, Fernandez Y, Castrillon E(2003)Removal of copper and cadmium ions from diluted aqueous solutions by low cost and waste material adorbents. Water Air and Soil Pollution142:357-373.
- Kounou GN, Nsami JN, Belibi DPB(2015) Adsorption of Zinc (II) ions from aqueous solution onto Kaolinite and Metakaolinite. Der Pharma Chemica7:51-58.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences