Relationship of Organic Acids to Reactive Oxygen Species and Oxidative Stress in Biochemistry and Drug Action
Peter Kovacic1* and Ratnasamy Somanathan2
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Center for Graduates and Research, Technological Institute of Tijuana, Apdo, Tijuana, Mexico
- *Corresponding Author:
- Peter Kovacic
Department of Chemistry and Biochemistry
San Diego State University
San Diego, CA, USA
Tel: +619-594-5595
E-mail: pkovacic@mail.sdsu.edu
Received Date: November 16, 2017; Accepted Date: December 10, 2017; Published Date: December 20, 2017
Citation: Kovacic P, Somanathan R (2017) Relationship of Organic Acids to Reactive Oxygen Species and Oxidative Stress in Biochemistry and Drug Action. J Cell Dev Biol Vol.1 No.1:9
Abstract
This report deals with involvement of organic acids, such as acetic, citric and lactic, in biochemistry and drug actions. In certain cases, there is participation in physiological action of electron transfer, reactive oxygen species and oxidative stress. Electron transfer agents include quinones, metals, ArNO2 and imines. Acids play important roles in transamination, electron transfer processes and antibiotic action. Much attention has been devoted to vinegar (acetic acid) as a condiment with health benefits.
Keywords
Organic acids; Reactive oxygen; Bioaction
Abbreviations
ET: Electron Transfer; ROS: Reactive Oxygen Species; OS: Oxidative Stress; FAD: Flavin Adenine Dinucleotide
Mini Review
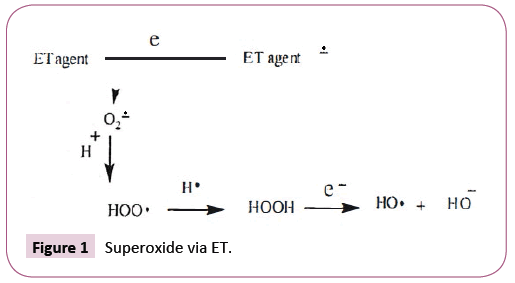
There are reports on involvement of organic acids in the action of drugs and biochemistry. However, there is rare discussion concerning the mode of action. This commentary provides evidence for the mechanistic role involving electron transfer (ET), reactive oxygen species (ROS), and oxidative stress (OS). Common ET agents are quinones, metal compounds, aromatic nitro compounds, and imines. Superoxide, generated via ET as illustrated in Figure 1, then serves as a precursor of other ROS.
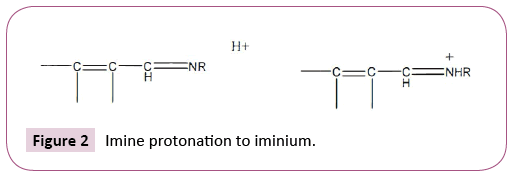
One example of imine-iminium ET agents involves protonation by acid of physiologically active conjugated imine species [1] (Figure 2).
There is increasing support for involvement of ET-ROS-OS in the mode of action entailing ET by iminium which is more active [1]. pK values are favorable for a number of bioacids, namely, citric (3.13), succinic (4.21) and acetic (4.76) [2]. The pK value involves the extent of dissociation from the required proton. The higher the dissociation, the greater the acidity. Participation of ET-ROSOS imines represents a novel application for organic acids.
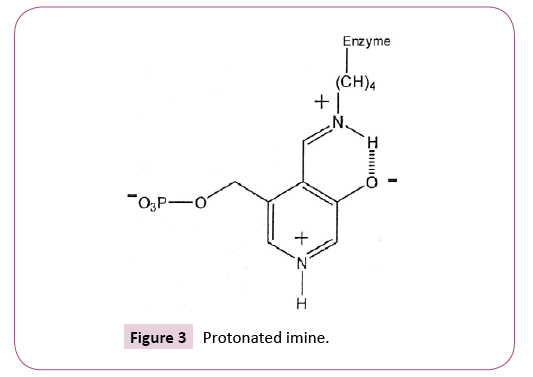
Acids are known to play important roles in various chemical processes, some of which may involve ET-ROS, whereas others may not. Transamination comprises conversion of the amine group in an amino acid to the corresponding keto acid [2]. The protonated imine (Schiff base) represents the first case of ET-ROS involvement in transamination (Figure 3).
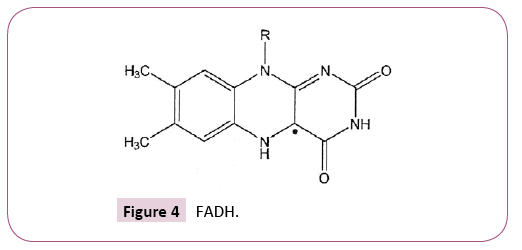
Glutathione reductase incorporates an ET prosthetic group Flavin adenine dinucleotide (FAD) which is a conjugated diminium type protonation of FAD, followed by electron uptake, yields FADH radical. The overall process comprises addition of the H (Figure 4).
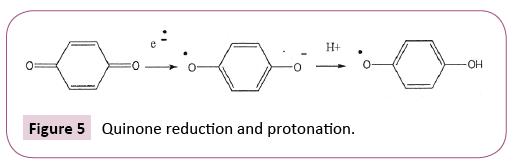
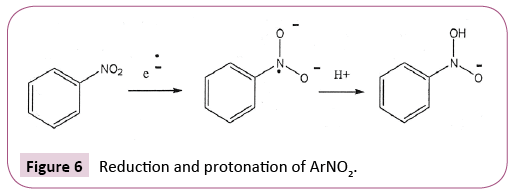
Involvement of acids with other ET agents is illustrated for quinones in Figure 5 and for aromatic nitro compounds in Figure 6.
A highly relevant article deals with organic acids as antibiotics [3]. The acids most involved are acetic (vinegar), citric, propionic, succinic, tartaric, malic and lactic. There is detailed discussion of mechanistic aspects. The acids are believed to function as antimicrobials by two modes of action. There is cytoplasmic acidification followed by uncoupling of energy generation and regulation, and build-up of the acid arises with resultant toxicity.
The cells of the bacteria react by attempting to expel the protons. An alternate to the theoretical aspects was advanced proposing uncoupling of electron transport involving oxidative respiration. Both theories provide satisfactory rationale for mode of action. The one involving electron transfer is somewhat similar to the ET-ROS-OS approach, which has previously been broadly applied as a mode of action for anti-infective agents. In addition, ETOS mechanisms may contribute to the biological effects of various antibiotic classes, including the inhibition of enzymes and metabolic processes [4]. The respiration aspect and energy uncoupling are related to mitochondria.
There is discussion of means of bacterial resistance to acids involving different signals to induce the synthesis of acid shock proteins among internal and external pH [5]. A number of related articles are available [6,7]. The role of acids and ET-ROS-OS in antibiotics represents a new finding.
Acknowledgement
Assistance by Thelma Chavez is appreciated.
References
- Kovacic P, Somanathan R (2014) New developments in the mechanism of drug action and toxicity of conjugated imines and iminiums, including related alkaloids. Open J Prevent Med 4: 583-597.
- Voet D, Voet J (1990) Biochemistry. (3rdedn), John Wiley and Sons, Canada.
- Mani-Lopez E, Garcia HS, Lopez-Melo A (2012) Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res lnternat 45: 713-721.
- Kovacic P, Becvar LE (2000) Modes of action of anti-infective agents: Focus on oxidative stress and electron transfer. CurrPharmaceut Des 6: 143-167.
- Kovacic P, Pozos RS, Somanathan R, Shangari N, O'Brien OJ (2005) Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure- activity relationships. Curr Med Chem 12: 2601-2624.
- Yildirim-Bicer AZ, Peker I, Akca G, Celik I (2014) In vitro antifungal evaluation of seven different disinfectants on acrylic resins. BioMed Research International 2014.
- Smulders FJM, Greer GG (1998) Integrating microbial decontamination with organic acids in HACCP programmes for muscle foods: prospects and controversies. lnternat J Food Microbial 44: 149-169.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences