Liver Iron Content by MRI at the start of hemodialysis
Patricia Carrilho1*, Ines Santiago2, Marta Alves3, Pedro Fidalgo4, Elsa Rodrigues5, Pedro Campos1 and Bruno Rodrigues1
1Hospital Professor Fernando Fonseca, Nephrology, Amadora, Portugal
2Champalimaud Foundation, Radiology, Lisboa, Portugal
3Centro Hospitalar de Lisboa Central, Epidemiology and Statistics Research Unit, Lisboa, Portugal
4Centro Hospitalar de Lisboa Ocidental, Nephrology, Carnaxide, Portugal
5Hospital Professor Fernando Fonseca, Radiology, Amadora, Portugal
- Corresponding Author:
- Patricia Carrilho
Department of Nephrology
Hospital Professor Doctor Fernando da Fonseca
IC 19, 2720-276 Amadora, Portugal
Tel: +351-917091587
Fax: +351-214345566
E-mail: patricia.s.carrilho@hff.min-saude.pt
Received date: July 05, 2017; Accepted date: July 22, 2017; Published date: July 30, 2017
Citation: Carrilho P, Santiago I, Alves M, Fidalgo P, Rodrigues E, et al. (2017) et al. Liver Iron Content by MRI at the Start of Hemodialysis. Adv kidney Dis Treat. Vol. 1 No. 2:9
Abstract
Introduction: Hepatic iron overload assessed by magnetic resonance imaging (MRI) has been described in the majority of chronic hemodialysis patients treated with intravenous (IV) iron, raising safety concerns. Whether iron overload is present in Chronic Kidney Disease (CKD) at the time of dialysis initiation is, however, unknown.
Methods: A prospective, observational, hospital-based study included 23 consecutive adult patients starting maintenance hemodialysis. Signal-intensityratio MRI was performed to calculate liver iron content (LIC). The association with clinical, biochemical markers of iron metabolism and prior anemia therapy was explored. MRI was repeated after 12 months in a cohort of 7 patients to explore LIC changes with time in hemodialysis under current anemia treatment.
Findings : At the start of hemodialysis, only 6 (26%) patients had normal LIC (<40 µmol/g), 14(61%)showedmild(40-100µmol/g) and3(13%)patientshadmoderate overload (101-200 µmol/g). None had severe overload. After 12 monthsin chronic hemodialysis underIV iron, LIC increased significantly,reachingmoderate overload in 6 patients and severe in one. Clinical, biochemical parameters and IV iron dose were not associated with the hepatic iron load detected in both occasions.
Conclusion: The results indicate that the majority of CKD patients have liver iron overload before initiating maintenance hemodialysis and that LIC increases steeply during the first year in dialysis with current anemia treatment.The observed major disturbance of iron homeostasis in CKD should be taken into account in newer approaches to anemia management in these patients.
Keywords
Chronic kidney disease; Hemodialysis; Hepatic iron load; Anemia
Abbreviations
BMI: Body Mass Index; CCI: Charlson Comorbidity Index; CHr: Reticulocyte Hb Content; CKD: Chronic Kidney Disease; CRP: C-Reactive Protein; DIOS: Dysmetabolic Iron Overload Syndrome; ESA: Erythropoesis Stimulating Agents; Hb: Haemoglobin; HIV: Human Immunodeficiency Virus; IV: Intravenous; LIC: Liver Iron Content; MRI: Magnetic Resonance Imaging; NAFLD: Non-Alcoholic Fatty Liver Disease; PBRC: Red Blood Cell Transfusions; PTHi: Intact Parathyroid Hormone; TSAT: Transferrin Saturation
Introduction
Intravenous iron therapy is increasingly used as part of the treatment of anemia in chronic kidney disease (CKD) and hemodialysis patients [1-3]. Recent Magnetic Resonance Imaging (MRI) studies [4-9] have shown that most maintenance hemodialysis patients receiving intravenous (IV) iron supplementation have moderate to severe hepatic iron overload, considered a reason for concern. It is unclear, however, how that hepatic overload develops, whether it is already present before iron therapy or hemodialysis start and why in the majority of patients undergoing hemodialysis, iron seems to be directly destined to the deposits with significant changes in ferritin levels, but without comparable changes in hemoglobin or transferrin saturation [10].
The present study was developed around three main questions:
1. Is there evidence of hepatic iron overload measured by MRI in patients before hemodialysis is started or any iron is administered?
2. How fast is the progression of hepatic iron overload in hemodialysis patients with current anemia treatment?
3. Would it be possible to identify at the start biomarkers that would enable to follow closely some patients at greater risk of developing iron overload?
This study aims to contribute to clarifying those questions by analysing liver iron content (LIC) by MRI in a sample of patients at the start of maintenance hemodialysis, repeating LIC measurement 12 months later and exploring the determinants of iron overload.
Methods
The study took place in Hospital Fernando da Fonseca after approval by its Ethical Review Board.
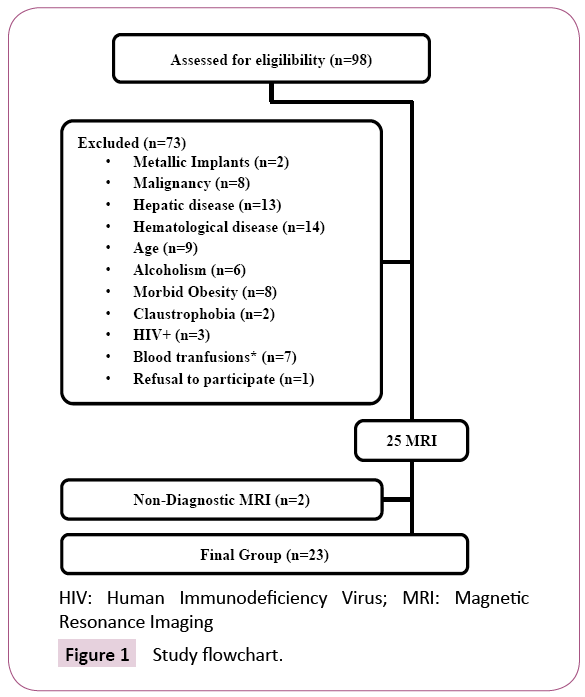
From March 2014 to February 2016, 98 consecutive patients at the initiation of maintenance hemodialysis were assessed for eligibility (Figure 1).
Criteria for exclusion were age (younger than 18 or older than 85 years old), more than 4 packed red blood cell transfusions (PBRC) in the last 12 months or any PBRC administration in the last 2 weeks, haematological or oncological disease, known genetic hemochromatosis, alcoholism, hepatic disease or infection with Human Immunodeficiency Virus (HIV).
Patients with morbid obesity, metallic devices or claustrophobia precluding MRI were also excluded.
After informed consent, 25 patients were enrolled in a prospective observational study consisting of hepatic MRI to measure LIC at the initiation of maintenance hemodialysis, retrieval of clinical data and biochemical blood analysis.
Two patients were not included in the analysis because of nondiagnostic MRI images due to respiration-induced artefacts, leaving 23 eligible patients.
MRI was postponed if IV iron therapy was administered in the previous week to minimize interference with MRI results [11].
Haemoglobin (Hb), Reticulocyte Hb Content (CHr), Transferrin Saturation (TSAT), ferritin, Intact Parathyroid Hormone (PTHi) and C-Reactive Protein (CRP) were determined.
Clinical data were collected, including demographic characteristics and history of previous transfusion, iron and erythropoesis stimulating agents (ESA) therapy in the last 36 months. Charlson comorbidity index (CCI) [12] and body mass index (BMI) [13] were calculated. In order to quantify alcohol consumption and identify active alcohol use disorders, patients were asked to complete confidentially AUDIT-C questionnaire [14].
After hemodialysis initiation, patients received routine hemodialysis treatments in outpatient chronic dialysis facilities, where anemia treatment was guided by regular evaluation of hematologic and biochemical markers of iron metabolism and adjusted according to nephrologist’s judgement, based on current practice guidelines.
Anemia treatment comprised once to thrice-weekly intravenous or subcutaneous administration of Epoetin beta (Recormon, Roche) or Darbepoetin alpha (Aranesp, Amgen) and iron sucrose (Venofer, Vifor International) in single-dose (100 mg) or divided doses (10 mg, 50 mg) thrice-weekly to once a month variable schedules.
Two patients were screened for HFE gene mutation (H63D and C282Y), after obtaining specific written informed consent for genetic analysis. Testing was performed by Genomed (Lisbon, Portugal), using real-time polymerase chain reaction direct and bidirectional sequencing.
After 12 months in routine hemodialysis, determination of LIC by MRI was repeated in 7 consecutive patients.
Last determination of Hb, ferritin, TSAT, total iron and ESA therapy were retrieved by enquiry from outpatient chronic dialysis facilities.
Magnetic resonance imaging was performed to calculate LIC in 1T equipment (Sigma, GE Medical Systems®, Milwaukee, WI) and consisted of 4 gradient echo acquisitions. Slice thickness: 10 mm; matrix: 128 × 256; repetition time/echo time/flip angle: 120/7/90°, 120/7/20°, 120/14/20°, 120/21/20°.
Scans were reviewed in consensus by two dedicated radiologists, who were blinded for clinical data. Oval regions of interest were placed in 3 lobe liver regions and right and left paraspinous muscles, each with a minimum area of 50 mm2. Mean signal intensity values were inserted in the University of Rennes’ online worksheet to calculate LIC based on the Gandon’s algorithm [15]. The upper limit of normal was set at 40 μmol/g [15,16]. Values between 40 and 100 μmol/g were considered to represent mild iron overload, values between 101 and 200 μmol/g moderate iron overload; and values >200 μmol/g severe iron overload [8].
Statistical analysis
Categorical data were presented as frequencies (percentages) and continuous variables as median and range (minimummaximum). Continuous variables were compared using the Mann-Whitney test given normality was not verified (Shapiro- Wilks test); categorical variables were compared using Fisher’s exact test. The association between the different variables and LIC on MRI was analysed using the Spearman correlation coefficient. To study the association between hepatic iron load (with two thresholds: 40 μmol/g and 50 μmol/g) and demographic factors, alcohol consumption, diabetes, previous iron or PBRC transfusions, logistic regression models were used. To compare initial and 12 months LIC values, Wilcoxon signed rank test was used. The level of significance α=0.05 was considered. Statistical analysis was performed using the Statistical Package for the Social Sciences for Windows 21.0 (IBM Corp. Released 2012. IBM SPSS Statistics for Windows. Armonk, NY: IBM Corp.).
Results
Characterization of patients
Patients’ median age was 66 years (27-83), 12 were male (52%). Seventeen were Caucasian (74%) and 6 were of African descent. The causes of kidney disease were diabetes (n=6; 26%), hypertension (n=5; 22%), glomerulonephritis (n=7; 30%), interstitial kidney disease (n=3; 13%) and unknown (n=2; 9%). Demographic and biochemical parameters at the start of HD are shown in Table 1.
| Gender/Age | Diagnosis | BMI | Hb (g/dL) | Ferritin (ng/ml) | TSAT (%) | CHr (pg) | LIC (µmol/g) | IV Iron | Oral Iron | PBRC | ESA | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M/64 | HTN | 28 | 9.5 | 7 | 8.0 | 23.0 | 5 | - | + | - | + | Normal |
| F/54 | DM | 24 | 11.5 | 77 | 16.0 | 32.0 | 25 | - | - | + | + | |
| F/72 | IKD | 42 | 8.2 | 321 | 25.0 | 30.6 | 25 | - | - | - | + | |
| M/76 | GN | 34 | 8.6 | 461 | 48.4 | 34.0 | 35 | - | - | - | - | |
| M/66 | IKD | 24 | 9.7 | 247 | 12.9 | 31.6 | 35 | - | - | - | - | |
| F/71 | DM | 24 | 7.9 | 344 | 14.2 | 27.4 | 35 | + | - | - | + | |
| F/41 | GN | 25 | 10.5 | 104 | 39.5 | 32.7 | 40 | + | - | - | + | Mild |
| M/68 | GN | 28 | 10.9 | 181 | 11.2 | 29.8 | 40 | - | - | - | - | |
| M/73 | GN | 24 | 7.5 | 602 | 13.2 | 28.7 | 45 | + | - | + | + | |
| M/27 | GN | 29 | 10.4 | 312 | 33.4 | 37.6 | 45 | - | - | - | + | |
| F/58 | GN | 40 | 8.2 | 139 | 10.1 | 32.2 | 50 | - | - | - | + | |
| F/55 | UK | 28 | 10.1 | 179 | 19.7 | 35.0 | 55 | - | - | - | + | |
| F/66 | DM | 36 | 10.5 | 56 | 13.4 | 33.2 | 60 | - | + | - | - | |
| M/49 | DM | 19 | 8.8 | 446 | 39.0 | 35.0 | 65 | + | + | + | + | |
| M/71 | HTN | 24 | 10.3 | 407 | 9.0 | 33.8 | 65 | - | - | - | - | |
| F/76 | UK | 17 | 8.0 | 140 | 17.3 | 28.4 | 75 | - | - | - | - | |
| F/78 | IKD | 29 | 8.4 | 167 | 10.2 | 27.7 | 80 | - | + | - | + | |
| M/83 | HTN | 27 | 9.7 | 156 | 26.8 | 31.2 | 80 | - | + | - | + | |
| M/69 | HTN | 35 | 9.0 | 199 | 10.9 | 33.2 | 85 | - | - | - | + | |
| F/54 | HTN | 28 | 10.5 | 216 | 35.0 | 33.0 | 100 | + | + | + | + | |
| F/56 | DM | 29 | 9.8 | 245 | 31.0 | 30.0 | 110 | + | - | - | + | Moderate |
| M/64 | DM | 27 | 10.5 | 579 | 31.0 | 34.7 | 160 | - | - | - | - | |
| M/76 | GN | 29 | 10.8 | 338 | 13.0 | 34.9 | 180 | - | - | - | - |
Gender: M-Male, F-Female; Age: years; HTN: Hypertension; DM: Diabetes Mellitus; IKD: Interstitial Kidney Disease; GN: Glomerulonephritis; UK: Unknown; BMI: Body Mass Index; Hb: Haemoglobin; TSAT: Transferrin Saturation; LIC: Liver Iron Content; IV: Intravenous; PBRC: Packed Blood Red Cell Transfusions
Normal values: Serum Ferritin 22-322 ng/ml; TSAT: 20-50%
Table 1: Patient demographics, biochemical parameters and LIC at the start of hemodialysis.
Liver iron content (LIC)
At the start of hemodialysis, only 6 out of 23 patients (26%) had normal LIC (<40 μmol/g) estimated by MRI (Table 1).
Of the remaining 17 patients, 14 (61%) had LIC values between 40 and 100 (mild overload) and 3 (13%) had LIC values between 101-200 μmol/g, representing moderate overload.
None of the demographic, clinical or biochemical parameters was associated with LIC at the start of hemodialysis (Tables 2 and 3).
| All patients (n=23) | Normal LIC group (n=6) <40 µmol/g |
Mild to moderate LIC group (n=17) ≥ 40 µmol/g |
p-value | |
|---|---|---|---|---|
| LIC (µmol/g) | 55.0 (5.0-180.0) | 30.0 (5.0-35.0) | 65.0 (40.0-180.0) | <0.001 |
| Age (years) | 66.0 (27.0-83.0) | 68.5 (54.0-76.0) | 66.0 (27.0-83.0) | 0.695 |
| Gender (M/F) | 12/11 | 3/3 | 9/8 | 1.000* |
| Caucasian (Y/N) | 17/6 | 3/3 | 14/3 | 0.279* |
| Diabetes (Y/N) | 6/17 | 2/4 | 4/13 | 0.632* |
| BMI (Kg/m2) | 28.0 (17.0-42.0) | 26.2 (24.0-42.0) | 28.0 (17.0-40.0) | 0.932 |
| CCI | 5.0 (2.0-9.0) | 5.0 (4.0-9.0) | 6.0 (2.0-9.0) | 0.912 |
| Audit-C score | 2.0 (0.0-6.0) | 1.5 (0.0-6.0) | 2.0 (0.0-4.0) | 0.827 |
LIC: Liver Iron Content; Gender: M- Male, F- Female; Y- Yes; N- No; BMI: Body Mass Index; CCI: Charlson Comorbidity Index Values are expressed as median and range (min-max); Gender, ethnicity and diabetes as number of individuals in each group *Fisher's Exact Test; remaining p-values are obtained by Mann-Whitney test
Table 2: Baseline demographic and clinical parameters analysis according to LIC subgroup.
| All patients (n=23) | Rho | p-value | Normal LIC group (n=6) <40 µmol/g |
Mild to moderate LIC group (n=17) ≥ 40 µmol/g |
p-value* | |
|---|---|---|---|---|---|---|
| Hemoglobin (g/dL) | 9.7 (7.5-12.0) |
0.183 | 0.403 | 9.1 (7.9-12.0) |
10.1 (7.5-11.0) |
0.342 |
| Ferritin (ng/mL) | 216.0 (7.0-602.0) |
0.176 | 0.422 | 284.0 (7.0-461.0) |
199.0 (56.0-602.0) |
0.973 |
| TSAT (%) | 16.0 (8.0-48.0) |
0.091 | 0.681 | 15.1 (8.0-48.0) |
17.3 (9.0-40.0) |
0.854 |
| CHr (pg) | 32.2 (23.0-38.0) |
0.315 | 0.143 | 31.1 (23.0-34.0) |
33.0 (28.0-38.0) |
0.131 |
| CRP (mg/dL) | 0.80 (0.00-9.90) |
-0.191 | 0.407 | 0.30 (0.00-5.20) |
1.30 (0.00-9.90) |
0.284 |
| PTHi (pg/ml) | 232.0 (41.0-456.0) |
-0.277 | 0.212 | 262.5 (200.0-417.0) |
195.5 (41.0-456.0) |
0.198 |
| Albumin (g/dL) | 3.1 (1.9-3.9) |
0.206 | 0.346 | 3.0 (2.3-3.6) |
3.1 (1.9-3.9) |
0.962 |
LIC: Liver Iron Content; Hb: Hemoglobin; TSAT: Transferrin Saturation; CHr: Reticulocyte Hemoglobin Content; CRP: C-Reactive Protein; PTHi: Intact Parathyroid Hormone
Values are expressed as median and range (min-max); Rho and p-value were obtained with Spearman’s correlation; p-value* was obtained with Mann-Whitney
Table 3: Baseline biochemical parameters at the start of hemodialysis and association with LIC.
As there are uncertainties of the measurement of low level of iron load with signal intensity ratio, we performed a logistic regression analysis with 2 thresholds: 40 μmol/g and 50 μmol/g (as set in previous work [8]) to study factors that may influence hepatic iron load, such as demographic factors, alcohol consumption diabetes and previous iron or PBRC transfusions, but none was statistically significant (Table 4).
| Parameter | Cut-off LIC 40 µmol/g | Cut-off LIC 50 µmol/g | ||||
|---|---|---|---|---|---|---|
| OR | 95% C.I. | p-value | OR | 95% C.I. | p-value | |
| Age | 0.97 | 0.89-1.05 | 0.460 | 1.04 | 0.97-1.11 | 0.318 |
| Gender | 0.89 | 0.14-5.72 | 0.901 | 1.20 | 0.23-6.18 | 0.827 |
| Diabetes | 0.61 | 0.08-4.70 | 0.640 | 2.25 | 0.32-15.76 | 0.414 |
| BMI | 0.96 | 0.82-1.12 | 0.603 | 0.94 | 0.81-1.09 | 0.433 |
| Audit-C | 0.96 | 0.55-1.66 | 0.873 | 0.78 | 0.47-1.30 | 0.343 |
| PBRC | 1.07 | 0.09-12.83 | 0.957 | 0.90 | 0.10-7.78 | 0.924 |
| IV Iron | 2.08 | 0.19-22.67 | 0.547 | 0.89 | 0.14-5.72 | 0.901 |
| Oral Iron | 2.08 | 0.19-22.67 | 0.550 | 7.14 | 0.68-75.22 | 0.102 |
| ESA | 0.92 | 0.13-6.56 | 0.930 | 0.52 | 0.09-3.03 | 0.472 |
| Iron+PBRC* | 1.00 | 0.99-1.00 | 0.688 | 1.00 | 0.99-1.00 | 0.630 |
BMI: Body Mass Index; Audit-C: 3-item alcohol screen; PBRC: Packed Blood Red Cell Transfusions; ESA: Erythropoetic Stimulating Agents Iron+PBRC*: An indicator was created to analyse the sum of total IV iron given (mg) and iron provided by PBRC transfusions, assuming that each pack provides around 200 mg of iron
Table 4: Univariable logistic regression analysis results for elevated LIC, according to two different LIC threshold, considering demographic, biochemical and treatment parameters.
Anemia treatment prior to hemodialysis start
No treatment: Six patients had never received any anemia treatment before the start of hemodialysis.
Their median LIC was 57 μmol/g (35-180).
Two male patients of the group without any anemia treatment already had LIC values of 160 and 170 μmol/g.
These two patients were screened for HFE gene mutation (H63D and C282Y).
Mutations were not detected, so Hereditary Hemochromatosis associated to HFE gene was not confirmed in these patients.
IV iron: Most patients (n=17, 74%) had not received IV iron previous to dialysis initiation.
In the group that had received previous IV iron, time from administration ranged from 2 weeks to 16 months before MRI (median time 1.25 months).
Intravenous iron was iron sucrose in all cases (ranging from 50 to 2800 mg, median dose 450 mg). All the patients who underwent IV iron administration (n=7) also did ESA.
LIC was not statistically different in patients who did IV iron (median 55 μmol/g range 35-110) and those who did not (median 55 μmol/g range 5-180), p=0.700) (Table 5).
| IV Iron | Oral Iron | PBRC | ESA | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-value | Yes | No | p-value | Yes | No | p-value | |
| LIC (µmol/g) | 55 (35-110) |
55 (5-180) |
0.700 | 72 (5-100) |
45 (25-180) |
0.420 | 55 (25-100) |
55 (5-180) |
0.903 | 50 (5-110) |
62 (35-180) |
0.518 |
| Hb (g/dL) | 9.3 (7.5-11.0) |
9.7 (80-12.0) |
0.440 | 9.6 (8.4-11.0) |
9.8 (7.5-12.0) |
0.860 | 9.9 (7.5-12.0) |
9.7 (7.9-11.0) |
0.839 | 9.5 (7.5-12.0) |
10.4 (8.0-11.0) |
0.184 |
| CHr (pg) | 31.3 (27.0-35.0) |
32.2 (23.0-38.0) |
0.506 | 32.0 (23.0-35.0) |
32.0 (27.0-38.0) |
0.503 | 32.5 (29.0-35.0) |
32.2 (23.0-38.0) |
0.776 | 32.0 (23.0-38.0) |
33.5 (28.0-35.0) |
0.383 |
| Ferritin (ng/mL) | 294.5 (104.0-602.0) |
181.0 (7.05-579.0) |
0.208 | 161.5 (7.0-446.0) |
247.0 (77.0-602.0) |
0.123 | 331.0 (77.0-602.0) |
199.0 (7.0-579.0) |
0.417 | 199.0 (7.0-602.0) |
292.5 (56.0-579.0) |
0.333 |
| TSAT % | 33.0 (13.0-40.0) |
13.4 (8.0-48.0) |
0.046 | 20.0 (8.0-39.0) |
16.0 (9.0-48.0) |
0.888 | 25.5 (13.0-39.0) |
14.2 (8.0-48.0) |
0.256 | 19.7 (8.0-40.0) |
13.2 (9.0-48.0) |
0.540 |
IV: Intravenous; PBRC: Packed Blood Red Cells; ESA: Erythropoesis Stimulating Agents; Hb: Hemoglobin; CHr: Reticulocyte Hemoglobin Content;
TSAT: Transferrin Saturation
Values are expressed as median and range (min-max); p-value was obtained with Mann-Whitney
Table 5: Association of Biochemical parameters at the start of hemodialysis with anemia treatment.
Six patients received oral iron (Table 1), their LIC was higher (median 72 μmol/g range 5-100) than those who did not receive it (median 45 μmol/g range 25-180). The difference, however, was not statistically significant (p=0.420).
Packed blood red cell transfusions: Four patients had previously received PBRC transfusions (a median dose of 2.5 packs, ranging from 1 to 3 packs, median time 4 months before MRI).
LIC was not different for those patients with previous PBRC transfusions (median 55 μmol/g range 25-100 vs. 55 μmol/g range 5-180).
Erythropoesis stimulating agents: Fifteen patients had already started ESA prior to starting HD of whom six had never received iron. ESA was darbepoetin alpha in 12 patients and epoetin beta in 3 patients. Median dose was 4000 UI/week (1000-16000), considering 1:200 darbepoetin alpha to epoetin beta ratio.
LIC was lower in patients already on ESA (median 50 μmol/g range 5-110 vs median 62 μmol/g range 35-180), but the difference was not statistically significant (Table 5).
IV Iron only affected TSAT significantly: TSAT was significantly higher in patients who did IV iron (33% range 13-40 vs. 13.4% range 8-48) p=0.046. Ferritin was higher in patients who had received IV iron (294.5ng/mL range 104.0-602.0 vs. 181.0 ng/ mL range 7.0-579.0) and PBRC (331.0 ng/mL range 77.0-602.0 vs. 199.0 ng/mL range 7.0-579.0) but the difference was not statistically significant. Hb and CHr did not differ in patients who had iron, PBRC or ESA (Table 5).
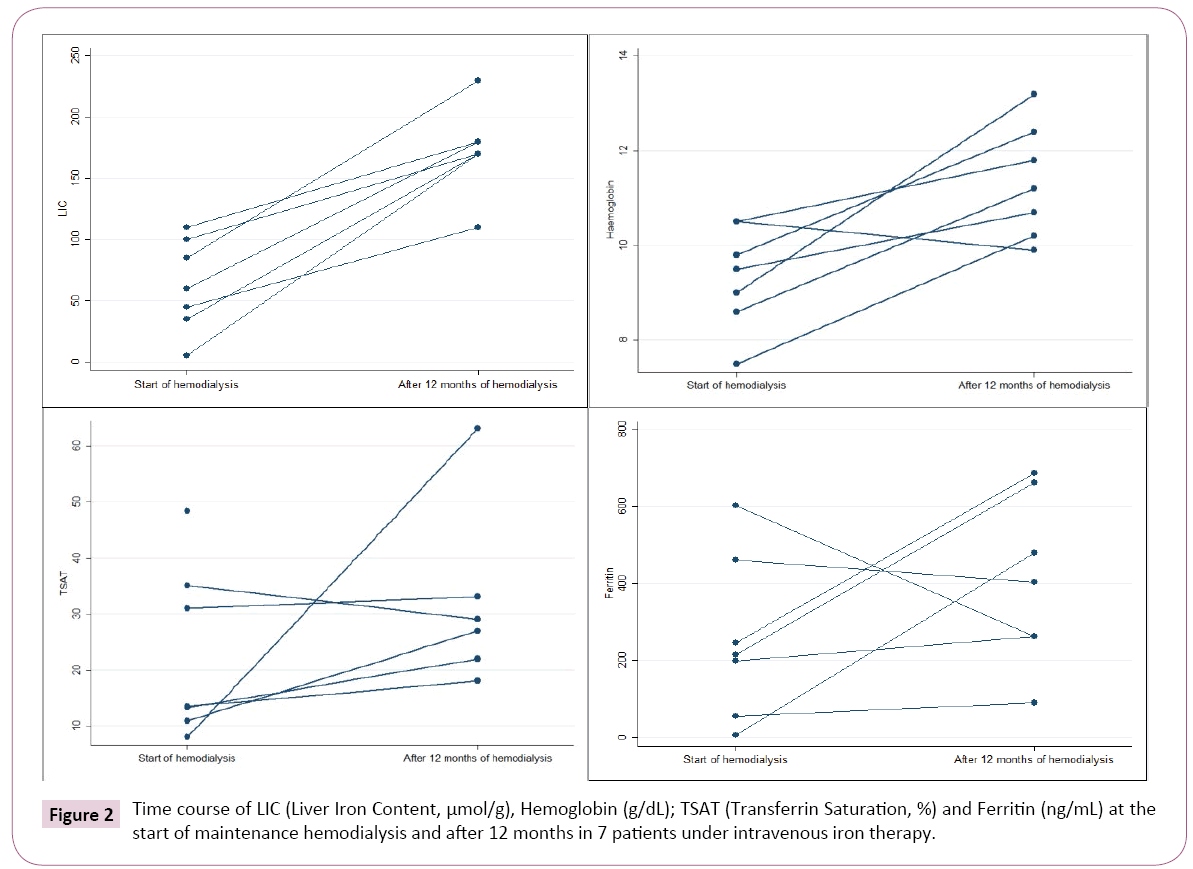
Liver iron overload at 12 months: After 12 months, 1 patient had died of sepsis due to limb ischemia. Eight consecutive patients repeated MRI to determine LIC. One of them was excluded due to non-diagnostic MRI.
In this group of patients (n=7), the initial LIC obtained at the start of hemodialysis had been moderate in 1 patient (>101-200 μmol/g), mild in 4 (≥ 40-100 μmol/g) and normal (<40 μmol/g) in the remaining 2 patients.
During the 12 months in chronic hemodialysis, all patients received IV iron (total minimum 1830 mg to maximum 5600 mg). Last IV iron administration was 9 to 34 days before MRI.
The repetition of MRI at 12 months showed a significant increase in LIC in all 7 patients. Six had moderate (>101 μmol/g) and 1 severe overload (>200 μmol/g) (Figure 2). In these 7 patients, LIC increased from 60 μmol/g (5-110) at the start of hemodialysis to 170 μmol/g (110-230), p=0.018 after 1 year.
There were no major blood losses reported and none had PBRC transfusions during 12 months follow-up. Hb, ferritin and TSAT at 12 months were retrieved (Figure 2). The increase in LIC did not correlate with the total dose of iron infused since the start of hemodialysis (p=0.702).
In an attempt to predict which patients are more prone to develop iron overload, we analyzed if there was any association between initial clinical and biochemical parameters and LIC at 12 months, but none was found.
Discussion
The present study was motivated by questioning if there is evidence of liver iron accumulation at the beginning of hemodialysis in CKD patients. The results show that there is.
In the 23 patients analyzed by MRI at the start of maintenance hemodialysis, only 6 had normal LIC at that management time-point, as quantified using the Gandon methodology [15]. Unexpectedly, mild to moderate hepatic iron overload was observed even in patients who had not received significant amounts of iron or any iron at all.
To our knowledge this is the first time that hepatic iron levels are seen to be abnormal already at the start of maintenance hemodialysis.
Hepatic MRI is considered nowadays the gold standard method for estimating and monitoring iron stores in iron overload disorders.
The method used (algorithm from Rennes University website) has a sensitivity of 89% and a specificity of 80% for iron overload disease [15].
Quantitative MRI for LIC estimation is based on the paramagnetic properties of iron. Iron particles cause local field in homogeneities that lead to a fast drop in signal intensity with increasing echo time in gradient echo imaging. This effect is proportional to iron concentration [16], so renal failure and its associated comorbidities are not expected to interfere with this effect per se.
Recently, a pilot study in 11 hemodialysis patients has shown that LIC determination based on signal-intensity-ratio MRI (Rennes University algorithm) accurately identifies iron load in hemodialysis patients, by comparison with liver histology [17].
The patients included in our study had a median low TSAT (16%) and high ferritin (216 ng/mL), meeting criteria for functional iron deficiency, characterized by impaired iron release from body stores that is unable to meet the demand for erythropoiesis (also called reticuloendothelial cell iron blockade) [18].
Not surprisingly, hepatic iron deposits in patients starting haemodialysis did not correlate with ferritin or TSAT, which once again proved to be unreliable as clinical tools to guide individual therapeutic decisions regarding iron administration [5].
Many patients screened originally to participate in the study could not be included; due to comorbid conditions that could cause iron overload (Figure 1). The small sample of patients evaluated represent a selection where iron overload observed is not expected, and could be attributed mainly to the pathogenesis of anemia of CKD.
Although multifactorial, the leading cause for CKD anaemia is considered to be insufficient erythropoetin (EPO) production, due to decreased EPO gene expression [18-20]. In uraemia, erythrocyte’s life cycle is known to be disturbed, featuring premature death, as well as of its precursors [19,21,22]. There is enhanced iron recycling, so, in the presence of chronic inflammation, we speculate that hepcidin’s role in restricting iron’s utilization could help explain the observed iron overload in the liver, in patients who did not receive previous iron supplementation.
In the present study hepcidin was not measured for the following reason: although known to be elevated in inflammation and CKD [23], its clinical utility has been undermined by its high intrapatient coefficient of variation [24].
In larger sample size studies [8] a positive correlation was found between LIC and hepcidin. Nevertheless, hepcidin’s elevation per se cannot explain iron overload in patients without any iron supplementation, as it also provokes a decrease in intestinal iron absorption, causing iron deficiency [25], as for example in patients with IRIDA, where a mutation in TMPRSS6 causes inappropriately high hepcidin levels.
Twenty six percent of the patients included in our study were diabetic, 65% overweight or obese, and these have frequently associated nonalcoholic fatty liver disease (NAFLD) and the metabolic syndrome, where increased deposition of iron in the liver could be a feature. Dysmetabolic iron overload syndrome (DIOS) is now a frequent finding in the general population [26]. Although elusive, its pathogenesis has been related to altered regulation of iron transport associated with steatosis, insulin resistance, and subclinical inflammation [27], which are highly prevalent in CKD patients. We speculate that the iron overload detected in the patients of our study may share some common pathway with DIOS.
The finding of iron overload already at the start of hemodialysis may call for clarification of the concept: “iron responsive” is not synonymous of “iron deficient”, as seems to be the case of these patients.
It may also lead to question the KDIGO recommendations of administering iron first rather than ESA in CKD not on dialysis patients, when TSAT is lower than 30% and ferritin levels are lower than 500 ng/mL [22,28].
In a cohort of 7 of those patients it was possible to observe the changes in liver iron concentration, ferritin and transferrin saturation a year after starting hemodialysis. Interestingly, hemoglobin levels were reached with considerable individual variation in ferritin and TSAT, which did not correlate with LIC or IV iron administered.
LIC increased significantly in all patients (Figure 2), in agreement with the findings of others in prevalent hemodialysis patients in other countries [5,7-9].
The steep rise in LIC suggests redundancy and limited effectiveness of IV iron, as much of the iron seems to be taken up and sequestered in the liver.
The reasons for such preference remain uncertain.
In this observational single-center study with a small size sample, it was not possible to identify at the start biomarkers for increased risk of iron overload.
Conclusion
In summary, the results indicate that the majority of CKD patients have liver iron overload before initiation of maintenance hemodialysis and that LIC increases steeply during the first year in dialysis with current anemia treatment.
The findings strengthen the view that iron administration to CKD patients should be done with caution, in line with the “plea for moderation” first put forward in an editorial of the American Journal of Medicine in 2012 [29].
In addition, it raises new questions regarding iron homeostasis in CKD and hemodialysis, outlining the need to find different therapeutic strategies in anemia of CKD.
Acknowledgement
We gratefully thank Maria de Sousa for her interest, encouragement and help in preparation of the manuscript.
Funding
PC acknowledges a travel grant from American Portuguese Biomedical Research Fund.
The present work was done with data acquired as part of the routine analysis of patients who initiate dialysis, funded therefore by the regular services of the Portuguese National Health Service.
Competing and Conflicting Interests
The authors have no conflicts of interest to disclose.
References
- Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, et al.(2013) Variation in intravenous iron use internationally and over time: The dialysis outcomes and practice patterns study (DOPPS). Nephrol Dial Transplant 28: 2570-2579.
- Miskulin DC, Zhou J, Tangri N, Bandeen-Roche K, Cook C, et al.(2013) Trends in anemia management in US hemodialysis patients 2004-2010. BMC Nephrol 14: 264.
- Freburger JK, Ng LJ, Bradbury BD, Kshirsagar AV, Brookhart MA (2012) Changing patterns of anemia management in US hemodialysis patients. Am J Med 125: 906-914.
- 4 Canavese C, Bergamo D, Ciccone G, Longo F, Fop F, et al.(2004) Validation of serum ferritin values by magnetic susceptometry in predicting iron overload in dialysis patients. Kidney Int 65: 1091-1098.
- Ferrari P, Kulkarni H, Dheda S, Betti S, Harrison C, et al. (2011) Serum iron markers are inadequate for guiding iron repletion in chronic kidney disease. Clin J Am Soc Nephrol 6: 77-83.
- Castillo NL, Boixadera H, Romeu M, Muñoz M, Jordi S, et al. (2016) Factors associated with the magnetic resonance imaging estimated liver concentration in long-term hemodialysis patientes receiving intravenous iron supplementation. Nephrol Dial Transplant 31: 2016.
- Holman R, Olynyk JK, Kulkarni H, Ferrari P (2016) Characterisation of hepatic and cardiac iron deposition during standard treatment of anaemia in haemodialysis. Nephrology.
- Rostoker G, Griuncelli M, Loridon C, Couprie R, Benmaadi A, et al. (2012) Hemodialysis-associated hemosiderosis in the era of erythropoiesis-stimulating agents: A MRI Study. Am J Med 125: 991-999.e1.
- Ghoti H1, Rachmilewitz EA, Simon-Lopez R, Gaber R, Katzir Z, et al. (2012) Evidence for tissue iron overload in long-term hemodialysis patients and the impact of withdrawing parenteral iron. Eur J Haematol 89: 87-93.
- Bailie GR, Larkina M, Goodkin DA, Li Y, Pisoni RL, et al. (2015) Data from the dialysis outcomes and practice patterns study validate an association between high intravenous iron doses and mortality. Kidney Int 87: 162-168.
- Rostoker G, Cohen Y (2014) Magnetic resonance imaging repercussions of intravenous iron products used for iron-deficiency anemia and dialysis-associated anemia. J Comput Assist Tomogr 38: 843-844.
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373-383.
- Centers of Disease Control (2011) Body mass index: Considerations for practitioners 4.
- Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA (1998) The AUDIT alcohol consumption questions (AUDIT-C). Arch Intern Med 158: 1789-1795.
- Gandon Y, Olivié D, Guyader D, Aubé C, Oberti F, et al. (2004) Non-invasive assessment of hepatic iron stores by MRI. Lancet 363: 357-362.
- Moirand R, Mortaji AM, Loréal O, Paillard F, Brissot P, et al. (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349: 95-97.
- Rostoker G, Laroudie M, Blanc R, Galet B, Rabaté B, et al. (2017) Signal-intensity-ratio MRI accurately estimates hepatic iron load in hemodialysis patients. Heliyon 3: e00226.
- Dongiovanni P, Fracanzani AL, Fargion S, Valenti L (2011) Iron in fatty liver and in the metabolic syndrome: A promising therapeutic target. J Hepatol 55: 920-932.
- Datz C, Felder TK, Niederseer D, Aigner E (2013) Iron homeostasis in the metabolic syndrome. Eur J Clin Invest 43: 215-224.
- Babitt JL, Lin HY (2012) Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631-1634.
- Almeida F, Santos S, Beirão I (2015) Regulation of erythropoietin production and recent trends in anaemia therapy. Port J Nephrol Hypert 29: 113-122.
- Ribeiro S, Belo L, Reis F, Santos-Silva A (2016) Iron therapy in chronic kidney disease: Recent changes, benefits and risks. Blood Rev 30: 65-72.
- Weiss G, Goodnough LT(2005) Anemia of Chronic Disease. N Engl J Med 35210352: 1011-1023.
- Fleming RE, Ponka P (2012) Iron overload in human disease. N Engl J Med 366: 348-359.
- Zaritsky J, Young B, Wang HJ, Westerman M, Olbina G, et al. (2009) Hepcidin--a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol 4: 1051-1056.
- Coyne DW (2011) Hepcidin: Clinical utility as a diagnostic tool and therapeutic target. Kidney Int 80: 240-244.
- Heeney MM, Finberg KE (2014) Iron-refractory iron deficiency anemia (IRIDA). Hematol Oncol Clin North Am 28: 637-652.
- Kidney Disease: Improving Global Outcomes (KDIGO) (2012) KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279-335.
- Vaziri ND (2012) Epidemic of iron overload in dialysis population caused by intravenous iron products: A plea for moderation. Am J Med 125: 951-952.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences