ISSN : 0976-8505
Der Chemica Sinica
Inhibition of Corrosion of Zinc in H2SO4 Medium by the Schiff Base, 4-Hydroxy Phenyl Methylidene-2-(1-Phenyl Ethylidene) Hydrazine Carbothioamide (4-HPMHC)
Onwu FK, Ogueji C and Mgbemena NM
Department of Chemistry, Michael Okpara University of Agriculture Umudike, PMB 7267 Umuahia Abia State, Nigeria
Abstract
Inhibitive effects of Schiff’s base, 4-hydroxy phenyl methylidene-2-(1-phenyl ethylidene) hydrazine carbothioamide (4-HPMHC) on the corrosion of zinc in H2SO4 at temperatures 303 K, 313 K and 323 K were studied using weight loss method. Inhibition efficiency of 4-HPMHC was found to depend on concentrations of inhibitor and the acid, temperature of the process and period of contact. The study revealed that 4-HPMHC inhibits the corrosion of zinc with maximum inhibition efficiency of 86.87% at 0.0005 M of the inhibitor. The kinetics of uninhibited and inhibited corrosion reaction was found to follow first order. Activation energy (Ea) value (18.22 J mol-1) of the inhibited corrosion system was higher than that of the uninhibited system with value of 4.919 J mol-1 at 0.0005 M of inhibitor and in 0.05 M of the H2SO4 acid. The calculated thermodynamic parameters viz; enthalpy, entropy and free energy of the inhibited reaction suggest that the process is spontaneous and exothermic. An adsorption characteristic of the studied inhibitor was best described by Langmuir adsorption isotherm.
Keywords
Adsorption; Corrosion inhibitor; Inhibition efficiency; Weight loss; 4-hydroxy phenyl methylidene-2-(1- phenyl ethylidene) hydrazine carbothioamide
Introduction
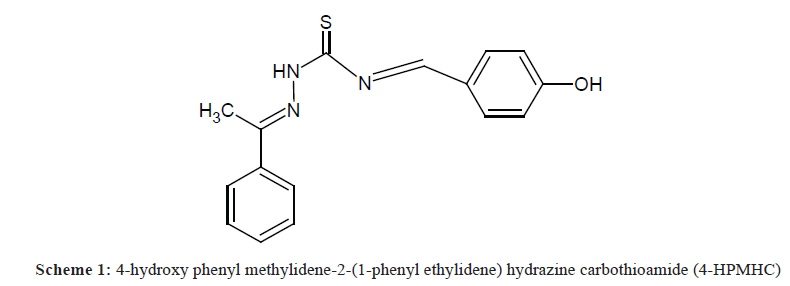
A very important process in the area of prevention and control of corrosion is the use of organic inhibitors. The introduction of organic inhibitors is in consonance with the focus of chemistry in the 21st century, which centers mostly on green chemistry. Organic inhibitors are found to be safer, eco-friendly and biocompatible when compared with their inorganic counterparts [1-3]. An important factor to be put into consideration in the mechanism of organic inhibitors when employed as corrosion inhibitors is the exclusive interaction between certain functionalities in the inhibitors with the active sites in the metallic surface. A corrosion inhibitor is generally referred to as a chemical substance that when applied in small quantities to a corrosive medium reduces the rate of corrosion of a metal or a metal alloy [4-7]. A search for a suitable inhibitor for the corrosion of metals involves a choice between those synthesized from cheap raw materials and those of organic origin containing hetero-atoms (N, O, S or P) in aromatic or long chain carbon compounds [8]. Schiff’s bases possess hetero-atoms such as nitrogen, oxygen and sulphur, which play major roles in this interaction by donating free electron pairs to the active sites of the metal surface. In addition, the presence of double bonds in Schiff’s bases makes them efficient inhibitors due the availability of π-electrons for interaction with metal surface. Compounds containing the aforementioned hetero-atoms in addition to the π-electrons have been found to display strong inhibition characteristics [9-12]. The present study seeks to use a synthesized Schiff’s base, 4-hydroxy phenyl methylidene-2-(1-phenyl ethylidene) hydrazine carbothioamide (4-HPMHC) to inhibit the corrosion of zinc in H2SO4 medium. The structure of the Schiff base used in the corrosion study is given in Scheme 1. From the structure, it is indicative that the 4-HPMHC molecule contains hetero-atoms and may be a good inhibitor for the corrosion of zinc in H2SO4.
Materials and Methods
Materials
The Schiff’s base (4-HPMHC) was synthesized by the method reported in Mandell et al. [13] and Hemandez-Molina et al. [14]. Zinc specimens used for the study were of dimensions 4 cm × 4 cm × 0.10 cm. The acid solutions (0.01 M to 2.0 M H2SO4) were prepared from analytical grade and were used for the weight loss measurement. The zinc coupon was washed in ethanol, dried in acetone and preserved in a desiccator. Various concentrations (0.0001 M to 0.0005 M) of the inhibitor, 4-HPMHC was also prepared.
Weight loss measurements
Weight loss measurements were carried out by immersing the zinc coupons in a beaker containing 150 mL of different concentrations (0.01 M to 0.05 M) of H2SO4 acid solutions and that containing the inhibitors at different times. Each set of experiment was conducted in a thermo stated water bath operated at temperatures 303 K, 313 K and 323 K. At the end of the contact time, each coupon was withdrawn from their respective solutions, washed in 5% chromic acid solution (containing 1% silver nitrate and 1% aluminium chloride), rinsed in boiling water and dried in acetone before weighing. From weight loss measurements, inhibition efficiency and degree of surface coverage were calculated using Eq. 1 and 2 respectively.
% I=1-W1/W2 × 100 (1)
θ=1-W1/W2 (2)
where, W1 and W2 are respectively the weight losses (g/dm3) of zinc in the presence and absence of inhibitor in H2SO4 solution. The corrosion rate of zinc in different concentrations of the acid was calculated from the weight loss data using the formula expressed by Eq. 3.
Corrosion rate=(weight loss × 87600)/(area × time × density) (3)
where, the weight loss is in grams, density of the specimen in g/cm3, area of the metal surface exposed in cm2 and time of immersion in hours.
Results and Discussion
Effects of concentration and temperature
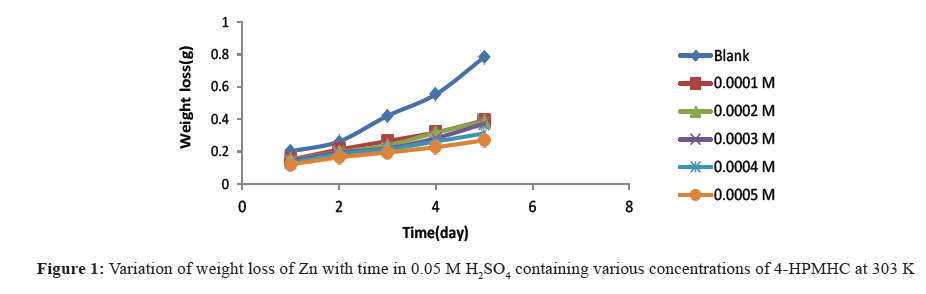
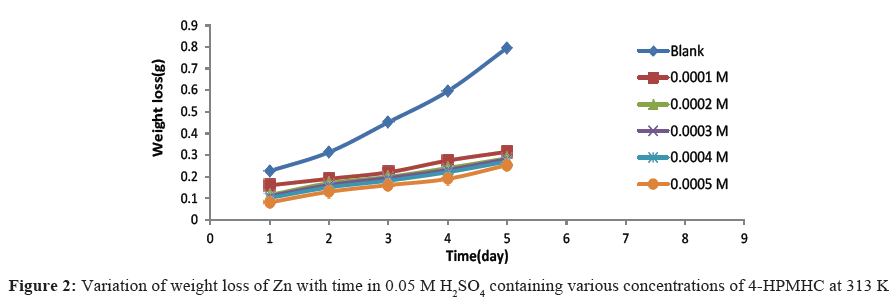
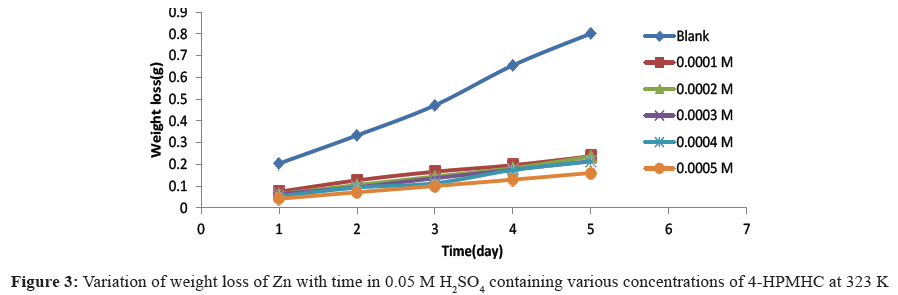
Figures 1-3 show the variation of weight loss with time for the corrosion of zinc in 0.05 M H2SO4 at temperatures 303 K, 313 K and 323 K, respectively. The plots showed that weight losses of the zinc in the acid increased with increase in temperature and with the period of contact, indicating that increased acid concentration and time of exposure aggravated the corrosion reaction. However, with the introduction of various concentrations of 4-HPMHC as inhibitor, the weight loss of zinc was found to decrease and this indicates that the inhibitor retarded the corrosion reaction of the zinc in H2SO4 and that the rate of corrosion decreased with the concentration of the inhibitor. Weight losses were also observed to decrease with temperature indicating that corrosion rate of the zinc is further lowered at higher temperatures and that 4-HPMHC is adsorbed on zinc electrode by chemical adsorption. This observation is in agreement with previous works that have been reported [15,16]. Table 1 shows the corrosion rate of zinc in H2SO4 and the inhibition efficiencies of 4-HPMHC for the corrosion process. Results showed that increase in concentration of the inhibitor, 4-HPMHC led to a decrease in corrosion rate (decrease in weight) of the zinc metal and a corresponding increase in inhibition efficiency of the 4-HPMHC. It was also found that there was no significant difference between values of inhibition efficiencies obtained at different concentrations of H2SO4 as shown in Table 1.
| 303 K | 0.01 M H2SO4 | 0.02 M H2SO4 | 0.03 M H2SO4 | 0.04 M H2SO4 | 0.05 M H2SO4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conc.(M) | CR ´ 10-3 | %I | CR ´ 10-3 | %I | CR ´ 10-3 | %I | CR ´ 10-3 | %I | CR ´ 10-3 | %I |
| 0.0001 | 7.64 | 49.90 | 7.81 | 48.79 | 7.50 | 50.79 | 7.42 | 51.32 | 7.59 | 50.19 |
| 0.0002 | 7.53 | 55.87 | 7.35 | 56.95 | 7.01 | 58.90 | 6.56 | 61.55 | 6.47 | 62.08 |
| 0.0003 | 7.27 | 61.98 | 6.94 | 63.71 | 6.91 | 63.82 | 6.14 | 67.86 | 5.87 | 69.23 |
| 0.0004 | 6.08 | 71.86 | 5.68 | 73.73 | 5.64 | 73.88 | 5.31 | 75.34 | 5.30 | 75.74 |
| 0.0005 | 5.26 | 77.51 | 4.17 | 82.17 | 4.09 | 82.49 | 4.00 | 82.88 | 9.36 | 83.16 |
| 303 K | 0.01 M H2SO4 | 0.02 M H2SO4 | 0.03 M H2SO4 | 0.04 M H2SO4 | 0.05 M H2SO4 | |||||
| Conc.(M) | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I |
| 0.0001 | 6.13 | 60.40 | 5.86 | 62.18 | 5.84 | 62.30 | 6.07 | 60.83 | 6.32 | 59.22 |
| 0.0002 | 5.55 | 68.46 | 5.86 | 66.69 | 5.67 | 67.79 | 5.46 | 68.96 | 5.45 | 69.02 |
| 0.0003 | 5.47 | 72.04 | 5.66 | 71.04 | 5.59 | 71.40 | 5.08 | 74.03 | 5.04 | 74.13 |
| 0.0004 | 5.25 | 76.85 | 5.45 | 75.99 | 5.15 | 77.32 | 5.06 | 77.70 | 5.03 | 77.85 |
| 0.0005 | 4.93 | 79.32 | 4.87 | 79.57 | 4.82 | 79.78 | 4.63 | 80.56 | 3.33 | 86.03 |
| 303 K | 0.01 M H2SO4 | 0.02 M H2SO4 | 0.03 M H2SO4 | 0.04 M H2SO4 | 0.05 M H2SO4 | |||||
| Conc.(M) | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I | CR x 10-3 | %I |
| 0.0001 | 4.62 | 70.14 | 5.34 | 65.76 | 5.45 | 65.05 | 5.46 | 65.00 | 5.65 | 63.81 |
| 0.0002 | 4.59 | 73.89 | 5.27 | 72.59 | 5.25 | 72.66 | 5.10 | 73.45 | 5.06 | 73.68 |
| 0.0003 | 4.18 | 78.61 | 5.14 | 75.39 | 5.25 | 74.84 | 4.97 | 76.19 | 4.82 | 76.92 |
| 0.0004 | 4.15 | 81.69 | 4.82 | 79.84 | 4.77 | 79.07 | 4.56 | 79.98 | 4.27 | 81.57 |
| 0.0005 | 3.13 | 86.87 | 4.01 | 83.09 | 4.34 | 82.08 | 4.04 | 83.34 | 3.76 | 84.50 |
Table 1: Corrosion rates (g cm-2 h-1) of zinc and inhibition efficiencies of 4-HPMHC for the corrosion of zinc in various concentrations of H2SO4
Kinetic and thermodynamic considerations
In order to determine the order and rate constants of the uninhibited and inhibited corrosion reaction of zinc, attempts were made to fit corrosion data obtained from weight loss measurements into different kinetic order of reactions. Results showed that the uninhibited and inhibited corrosion reactions of zinc obeyed first order kinetics at all temperatures studied. A first order equation is written as:
d([Zn]0-x)/dt=k1t (4)
where, [Zn]0-x is the weight loss of zinc, k1 is the first order reaction rate constant and t is the time in hours.
Rearranging and integrating Eq. 4, yields Eq. 5:
-log([Zn]0-x)/[Zn]0=k1t/2.30 (5)
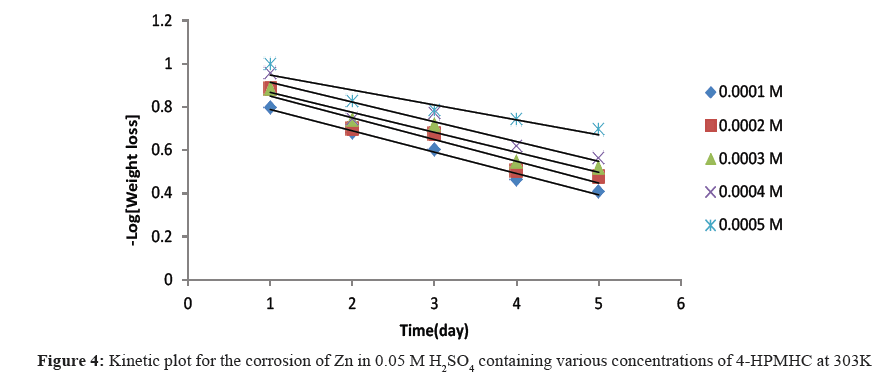
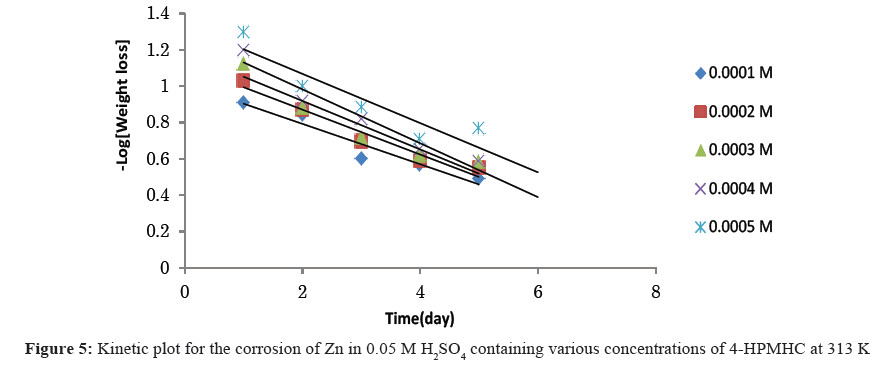
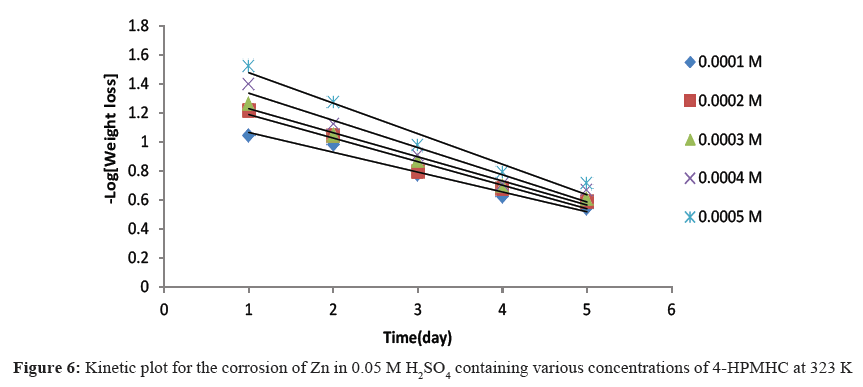
From Eq. 5, a plot of -log[weight loss of zinc] versus time should produce a straight line with slope equals to k1/2.303. The half-lives of the inhibited and uninhibited corrosion reactions were also calculated using Eq. 6 [17,18].
t½=0.693/k1 (6)
Figures 4-6 show kinetic plots for the corrosion of zinc in 0.05 M H2SO4 in the presence of different concentrations of 4-HPMHC. From the slopes of the plots in Figures 4-6, values of k1 were calculated. Values of t½ were also calculated using Eq. 6. From the calculated values of t½, it was found that 4-HPMHC has the tendency of extending the half-life of zinc corrosion in H2SO4.
In order to calculate the activation energy of the corrosion reaction of zinc in the H2SO4 (in the absence and presence of 4-HPMHC), the Arrhenius equation was used (Eq. 7) [17,18].
CR=Aexp(-Ea⁄RT) (7)
where, A is Arrhenius frequency or pre-exponential factor, CR is the corrosion rate, Ea is the activation energy of corrosion, R is the gas constant and T is the absolute temperature (K). On linearizing Eq. 7, Eq. 8 is obtained.
log k=log A-Ea⁄2.303RT (8)
From Eq. 8, a plot of log k versus 1/T should produce a straight line with slope equal to -Ea/2.303R and intercept equal to log A (plots of log k versus 1/T not shown). From the slope of lines on the plots, the calculated values of Ea which ranged from 6.326 J mol-1 to 25.02 J mol-1 (as shown in Table 2), show that the activation energy of the inhibited corrosion reaction is higher than that of uninhibited and further indicates that the 4-HPMHC inhibits the corrosion of zinc in the H2SO4 medium.
| Conc.(M) | Ea (J/mol) | ΔHads (J/mol) | ΔS (J/mol/K) | ΔGads (kJ/mol) at 303 K | |
|---|---|---|---|---|---|
| 0.01M H2SO4 | 0.0001 | 15.16 | -23.51 | 72.88 | -22.11 |
| 0.0002 | 20.24 | -23.19 | 58.82 | -17.85 | |
| 0.0003 | 20.57 | -25.60 | 58.11 | -17.53 | |
| 0.0004 | 21.21 | -18.48 | 53.31 | -16.17 | |
| 0.0005 | 22.62 | -24.16 | 50.70 | -15.39 | |
| 0.02 M H2SO4 | 0.0001 | 6.326 | -18.44 | 120.2 | -36.44 |
| 0.0002 | 6.695 | -16.44 | 102.1 | -30.95 | |
| 0.0003 | 12.24 | -15.10 | 84.86 | -25.73 | |
| 0.0004 | 13.60 | -9.440 | 80.88 | -24.52 | |
| 0.0005 | 15.57 | -3.293 | 74.69 | -22.63 | |
| 0.03 M H2SO4 | 0.0001 | 6.987 | -15.93 | 101.2 | -30.68 |
| 0.0002 | 11.24 | -14.04 | 88.23 | -26.75 | |
| 0.0003 | 11.79 | -9.669 | 86.47 | -26.31 | |
| 0.0004 | 19.09 | -17.36 | 82.66 | -25.06 | |
| 0.0005 | 25.02 | -14.62 | 76.40 | -23.16 | |
| 0.04 M H2SO4 | 0.0001 | 6.326 | -2.211 | 123.3 | -37.36 |
| 0.0002 | 6.695 | -8.999 | 102.9 | -31.19 | |
| 0.0003 | 12.24 | -11.43 | 95.85 | -29.05 | |
| 0.0004 | 13.60 | -13.67 | 91.07 | -27.61 | |
| 0.0005 | 15.57 | -15.42 | 84.36 | -25.58 | |
| 0.05 M H2SO4 | 0.0001 | 8.101 | -14.98 | 114.9 | -34.83 |
| 0.0002 | 8.866 | -12.85 | 97.40 | -29.53 | |
| 0.0003 | 10.05 | -10.88 | 94.28 | -28.58 | |
| 0.0004 | 12.14 | -11.65 | 91.72 | -27.80 | |
| 0.0005 | 18.22 | -4.499 | 86.06 | -26.07 |
Table 2: Thermodynamic parameters for the corrosion of zinc in 0.01 M to 0.05 M H2SO4 in the presence of different concentrations of the inhibitor, 4-HPMHC
In order to calculate thermodynamic parameters for the corrosion reaction, transition state equation (Eq. 9) was used [17,18].
K=RT/Nh exp(ΔS/R)exp(-ΔH/RT) (9)
On rearranging and taking the logarithm of both sides of Eq. 9, Eq. 10 is obtained.
log (K/T)=log R/Nh+ΔS/2.303R-ΔH/2.303RT (10)
where, ΔHads is the enthalpy of adsorption, ΔSads is the entropy of adsorption, R is the gas constant, T is the temperature (K), N is the Avogadro’s number and h is the Planck’s constant. From Eq. 10, a plot of log K/T versus 1/T should give a straight line with slope equal to -ΔH/2.303R and intercept equal to log R/Nh+ΔS/2.303R. The thermodynamic parameters, ΔH and ΔS were calculated from the slope and intercept of the linear plots (plots not shown) and presented in Table 2.
Values of ΔHads (Table 2) calculated from slopes of lines of the plots ranged from -2.211 J mol-1 to -25.60 J mol-1 for the different concentrations of the inhibitor investigated and with an average value of -14.23 J mol-1. These negative values clearly indicate the exothermic nature of the inhibited corrosion reaction. Values of ΔSads calculated from intercepts of lines on the plots ranged from 50.70 J mol-1 to 123.3 J mol-1 with an average value of 86.93 J mol-1 K-1. The decreasing values of ΔSads at higher concentrations of the inhibitor indicate that the adsorption of 4-HPMHC on the surface of zinc is accompanied by decreasing disorderliness.
Values of free energy of adsorption ΔGads were calculated by substituting values of ΔHads and ΔSads into Eq. 11.
ΔG=ΔH-TΔS
Values of ΔGads calculated from Eq. 11 ranged from -30.16 J mol-1 to -17.83 J mol-1 for different concentrations of inhibitors investigated. The negative values obtained indicate that the adsorption of 4-HPMHC on zinc surface is spontaneous.
Adsorption consideration
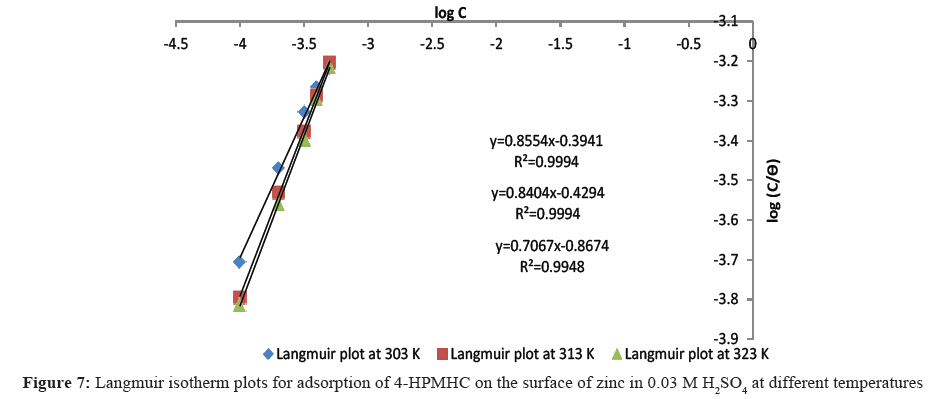
Adsorption isotherm provides a clue to the mode and mechanism of adsorption. Attempts were made to fit data obtained from weight loss measurement into different adsorption isotherm models including those of Langmuir, Frumkin, Freundlich, Temkin, and Florry Huggins isotherms. Results show that experimental corrosion data fitted best into Langmuir adsorption isotherm. The Langmuir adsorption isotherm is expressed by Eq. 12.
C/θ=1/K+C (12)
where C is inhibitor’s concentration, θ is the degree of surface coverage and K is the Langmuir adsorption constant. Taking the logarithm of both sides of Eq. 12, Eq. 13 is obtained.
log (C⁄θ)=log C-log K (13)
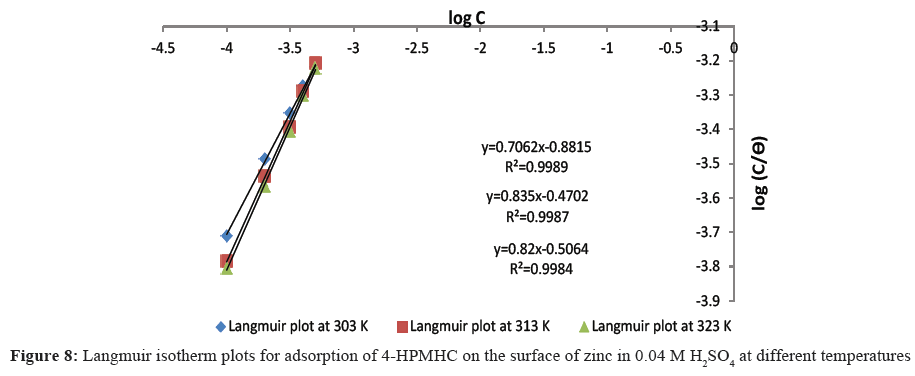
From Eq. 13, a plot of log(C⁄θ) versus log C should give a straight line if the assumptions of Langmuir are valid [19]. Langmuir plots for the inhibited corrosion reaction at two different concentrations of the H2SO4 acid are shown in Figures 7 and 8. The high coefficient of correlation (R2) values which ranged from 0.994 to 0.999 confirm that Langmuir isotherm best correlated the experimental corrosion data. The applicability of Langmuir adsorption isotherm to the adsorption of 4-HPMHC on zinc coupons suggests strong adsorption of the inhibitor on the zinc surface and the existence of monolayer of the adsorbed inhibitor. This clearly shows that there are interactions between the active sites on the zinc surface and inhibitor species leading to the formation of spread films on the zinc surface and which consequently inhibited the corrosion process. Similar observations have been reported [20-22].
Conclusion
From the results of the study carried out, the following conclusions can be drawn:
• 4-HPMHC is a good inhibitor for the corrosion of zinc in 0.01 M to 0.05 M concentration of H2SO4.
• Inhibition efficiency of 4-HPMHC is dependent on concentration, temperature and period of exposure.
• Inhibited corrosion reaction of the Zn metal in the presence of 4-HPMHC follows first order kinetics.
• Thermodynamic parameters calculated show that adsorption of 4-HPMHC on zinc surface is exothermic, spontaneous and is accompanied by decreasing disorderliness.
• The adsorption of the 4-HPMHC on zinc surface proceeds via chemical adsorption mechanism and the adsorption process could best be described by Langmuir adsorption isotherm.
References
- Kumpawat V, Garg U, Tak RK (2009) Corrosion Inhibition of aluminium in acid media by naturally occurring plant Artocarpus heterophyllus and Acacia Senegal. J Ind Council Chem 26: 82.
- Chauhan R, Garg U, Tak RK (2011) Corrosion inhibition of aluminiumin acid media by Citrullus Colocynthis extract. J Chem 8: 85-90.
- Obot IB, Umoren SA, Obi-Egbedi NO (2011) Corrosion inhibition and adsorption behaviour for aluminuim by extract of Aningeria robusta in HCl solution: Synergistic effect of iodide ions. J Mater Environ Sci 2: 49-60.
- James AO, Oforka NC, Abiola K (2007) Inhibition of Acid Corrosion of Mild Steel by Pyridoxal and Pyridoxol Hydrochlorides. Int J Electrochem Sci 2: 278-284.
- Ebenso EE, Okafor PC, Ekpe UJ, Ibok UJ, Onuchukwu AI (2004) The joint effects of halide ions and methylene blue on the corrosion inhibition of aluminium and mild steel in acid corrodent. J Chem Soc Nig 29: 15-25.
- Ebenso EE (2001) Inhibition of corrosion of mild steel in HCl by some Azo dyes. Nig J Chem Res 6: 8-12.
- Ekpe UJ, Okafor PC, Ebenso EE, Offiong OE, Ita BI (2001) Mutual effects of TSC derivatives on the acidic corrosion of aluminium. Bull Electrochem 17: 131-135.
- Muralidharan S, Chandrasek R, Iyer SK (2000) Effect of piperidones on hydrogen permeation and corrosion inhibition of mild steel in acidic solutions. Proc Indian Acad Sci 112: 127-136.
- Upadhyay RK, Mathur SP (2007) Effect of Schiff's Bases as Corrosion Inhibitors on Mild Steel in Sulphuric Acid. J Chem 4: 408-414.
- Chitra S, Parameswari K, Selvaraj A (2010) Dianiline Schiff Bases as Inhibitors of Mild Steel Corrosion in Acid Media. Int J Electrochem Sci 5: 1675-1697.
- Desai MN, Talati JD, Vyas CV, Shah NK (2008) Some Schiff bases as corrosion inhibitors for zinc in sulphuric acid. Ind J Chem Technol 15: 228-237.
- Devender KM, Rajesh KU, Alok C (2010) Study of corrosion inhibition efficiency of some Schiff’s bases on aluminium in trichloroacetic acid solution. Rev Roun Chim 55: 227-232.
- Mandell BA, Meredith TA, Aguilar E, El-Massry A, Sawant A, et al. (1992) Sparfloxacin and other new fluoroquinolones. J Antimicrob Chem 30: 739-744.
- Hemandez-Molina R, Mederos A (2003) Acyclic and Macrocyclic Schiff Base Ligands" inComprehensive Coordination Chemistry, Elsevier-pergamon press, Oxford and New York.
- Shokry H, Yuasa M, Sekine I, Isah RM, El-Baradie HY (1998) Corrosioninhibition of mild steel by Schiff base compounds in various aqueous solutions. Corros Sci 40: 2173-2186.
- Ehteshamzade M, Shahrabi T, Hosseini MG (2006) Inhibition of copper corrosion by self-assembled films of new Schiff bases and their modification with alkanethiols in aqueous medium. Appl Surf Sci 252: 2949-2959.
- Abdallah M (2004) Antibacterial drugs as corrosion inhibitors for corrosion of aluminum in hydrochloric solution. Corrosion Sci 46: 1981-1996.
- Abdallah M (2002) Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros Sci 44: 717-728.
- Ashassi-Sorkhabi H, Gbalebsaz-Jeddi N (2005) Inhibition effect of polyethylene glycol on the corrosion of carbon steel in sulphuric acid. Mat Chem Phys 92: 480-486.
- Orubite-Okorosoye K, Oforka NC (2004) Corrosion Inhibition of Zinc on HCl using Nypa fruticans Wurmb Extract and 1,5 Diphenyl Carbazone. J Appl Sci Environm Mgt 8: 57-61.
- Shetty SD, Shetty P, Nayak HV (2006) Inhibition of mild steel corrosion in acid media by n-(2-thiophenyl)-n/-phenyl thiourea. J Chilean Chem Soc 51: 849- 853.
- Atkins PW (1991) A textbook of physical chemistry, Oxford University Press, New York.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences