ISSN : 0976 - 8688
Der Pharmacia Sinica
Formulation and Optimization of Delayed Release MUPS (Multiple Unit Particulate System) Tablets of Omeprazole
Sundar VD*, Nandhakumar S, Alekya CH and Dhanaraju MD
Department of Pharmaceutics, GIET School of Pharmacy, Rajahmundry, Andhra Pradesh, India
Abstract
The work was aimed to persuade the effect of size of sugar spheres and compression parameters on the prepared tablets, containing enteric coated pellets of omeprazole. Multiple unit dosage forms of Omeprazole were formulated by wurster process. The sugar spheres (#18/20, #20/25, # 30/35, #40/60) were coated with the drug, HPMC 5cps, Eudragit L30D55 and then with PEG. The pellets were compressed into tablets using Prosolv granules as a pressure absorbing matrix. The compressed tablets were characterized for DSC, SEM, and XRD. The Optimized formulations showed plastic deformation and the pellets embedded in the tablet maintain their integrity with no considerable change in their surface properties. In vitro release profile of formulation F8 containing sugar spheres (#40/60) coated with, Eudragit L30D55 70% w/w and PEG 4000 20% w/w as cushioning agent showed release up to 97% at the end of 1 hr in buffer. The DSC, X-RD results proves that there is no potent incompatibility between the drug and the polymer. The higher the size of sugar spheres higher will be the smash up to the coating and less resistance to gastric environment than the small spheres. Small pellets were best suited for compression of multiple unit particulate systems of Omeprazole.
Keywords
Omeprazole, Sugar spheres, Eudragit L30D55, Prosolv
Introduction
Compression of pellets and microspheres into compact tablets is a smart approach to prepare a single unit dosage forms that will readily crumble into its essential components when exposed to gastric fluids [1]. These dosage forms will maintain the advantages of pellets being a single unit dosage form [2,3] i.e., spread uniformly throughout the gastro intestinal tract, improves bioavailability maximize the drug absorption; minimize local irritation of drug which indicates pellets can be used for immediate drug delivery [4-6]. The multiparticulates can be filled into capsules or compressed into tablets. The fabrication of multiparticulates into tablets is proven to be familiar than the hard gelatin capsules where the later have been tampered, patient incompliance, difficulty in oesophageal transport, high cost production [7-9].
Pelletized tablets put forward various advantages like large dose drug administration, increased production rate, reduced possibility of local irritation, toxicity, expected bioavailability, minimum fluctuations in plasma concentration of drug caused by food effects. Fabrication of compressed tablets using coated pellets is a tough task as; the pellets should not fuse into non disintegrating matrix during compaction, and the drug release not to be altered by the compaction process. With reservoir type pellet dosage forms, the layer of coating applied must withstand the compression force; it can distort, but should not burst or must still provide controlled release even if ruptured [10].
The various parameters taken into consideration during compression of pellets are pellet core, nature of the polymer, size of pellets, nature of excipients etc. The pellet size influences the compaction properties and release of encapsulated drug from the compacted pellets. Pellets of smaller size have been found to be less affected than pellets of larger one [11]. As the particle size increases, the damage to coating simultaneously increased this exhibit maximum difference between the release profiles of compressed and uncompressed pellets [12]. Rate retarding polymers used in the coating should be highly rigid and flexible to be able to become accustomed the deformation of the pellets without rupture [13,14]. The films designed from acrylic polymers are more flexible and therefore more appropriate for the coated pellets to be compressed into tablets [15]. Among the acrylic polymers Eudragit L30 D55 resist the compaction forces better when compared with the uncoated pellets [16]. An additional coating of soft waxy materials such as Polyethylene glycol, poly ethylene oxides increases the deformation susceptibility of the pellets [17]. Excipients also affect the compression of enteric coated pellets into tablet and their release profiles. Weight variation and drug content uniformity was minimized by using large particle size fractions of MCC as the filler [18]. Lower damage to the coating was achieved by using the maximum amount of compacted granules and rest of excipients as finely divided powder. A combination of 50% of MCC and remaining excipients was found to be most suitable for minimizing the damage to the coating [19].
Omeprazole a acid labile H++ k+ ATPase inhibitor also known as gastric proton pump inhibitor (Rowasa). It is a substituted benzimidazole and a weak base with a Pka of 3.97. It degrades rapidly in the acidic aqueous solutions. It is having a degradation half life of 10 min at PH values lower than 4 [20]. To overcome the stability problems of omeprazole the best approach appears to be application of an enteric coating on to the solid dosage form [21].
Materials and Methods
Omeprazole was received as gift Sample from Zydus cadila health care ltd (India), Sugar Spheres from Signet pharma (India), (HPMC) Hydroxy Propyl Methyl Cellulose 5 cps (Colorcon, Goa, India), Sodium Hydroxide from Rankem chemicals, India, {(MCC) Micro Crystalline Cellulose+Aerosil)} Prosolv HD 90, Prosolv SMCC 90 from JRS Pharma, Patterson, NY, Talc and Polysorbate 80 (Merck Specalities pvt ltd, India), Triethyl Citrate, Poly Ethylene Glycol 4000 and Sodium Stearyl Fumarate from Micro Organ Chem. (Mumbai, India), Crosspovidone XL (Shanghai Shenmei Pharmaceutical Technology Co., Ltd. China).
Preparation of pellets by fluid bed processing technique
A weighed amount of omeprazole was dispersed into the HPMC dispersion containing talc as anti tacking agent. The PH of the dispersion was adjusted to 10-12 by using 0.1 N NaOH. The drug dispersion was applied as fine mist on to the sugar spheres (#18/20, #20/25, #30/35, #40/60) placed in to the chamber of an aromatic fluid bed spray coater(plam glatt GPCG 1.1) with a wurster column insert and fluidized to equilibrate to the temp of 32°C. The spheres were film coated with HPMC and talc dispersion which prevents the reaction between basic drug and acidic enteric coating polymer. Eudragit L30 D55 was accurately weighed and added to the half of the amount of deionized water. The remaining water was used to prepare talc and TEC dispersion. The dispersion was added to the Eudragit dispersion under gentle stirring. The dispersion was coated on film coated spheres to obtain different percents of coatings. An extra coating layer of cushioning agent containing PEG 4000, tween 80, talc is applied to overcome the cracks formed during compression. The outlet temperature was adjusted to 35°C. The atomization was set at 1 bar. The compositions of the coating dispersions were presented in Table 1.
| Ingredients | B1 (#18/20) | B2 (#20/25) | B3 (#30/35) | B4 (#40/60) | ||||
|---|---|---|---|---|---|---|---|---|
| Drug loading | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
| Sugar spheres | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| Omeprazole | 40 | 40 | 40 | 40 | 40 | 40 | 40 | 40 |
| HPMC 5cps | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 |
| Sodium hydroxide | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
| Purified Water | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
| Sub coating | ||||||||
| HPMC 5cps | 17.86 | 17.86 | 17.86 | 17.86 | 17.86 | 17.86 | 17.86 | 17.86 |
| Talc | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 | 0.94 |
| Purified Water | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
| Enteric coating | ||||||||
| Eudragit L30D55 | 159.64 | 223.49 | 191.56 | 223.49 | 191.56 | 223.49 | 191.56 | 223.49 |
| Tri ethyl citrate | 4.79 | 6.71 | 5.75 | 6.71 | 5.75 | 6.71 | 5.75 | 6.71 |
| Talc | 3.67 | 5.13 | 4.39 | 5.13 | 4.39 | 5.13 | 4.39 | 5.13 |
| Water | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
| Cushion coating | ||||||||
| PEG4000 | 28.74 | 19.18 | 19.18 | 19.18 | 19.18 | 19.18 | 19.18 | 19.18 |
| Polysorbate 80 | 8.46 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 |
| Talc | 8.46 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 | 9.58 |
| Purified water | Qs | Qs | Qs | Qs | Qs | Qs | Qs | Qs |
Table 1: Weight percentage compositions of different formulations for pelletization of omeprazole
Acid resistance of pellets
A gastro resistant study was performed using apparatus II of USP (Electrolab, Mumbai) at 100 rpm/min in simulated gastric fluid at 37°C for 2 hrs. The test was run in gastric fluid for 2 hrs. At the end of 2nd hr, the pellets were removed from the vessel, media was discarded, the pellets were crushed and powder equivalent to 40 mg of drug was transferred to a volumetric flask. Required amount of 0.1 N NaoH was added, mixed, filtered. The filtrate was suitably diluted with 6.8 phosphate buffer and analysed by UV spectrophotometer at 305 nm.
Granulation of excipients
Compression process was performed using prosolv, crosspovidone XL and sodium stearyl fumarate in order to compare the particle size of excipients with the pellets. The blend was forced through a roller compactor with 3 rpm, 20 km hardness. The compressed compacts were further passed through co mill (40 G, 30 Hz) to obtain the granular blend. With the help of laboratory size V-shaped blender, the components of pellets viz. coated pellets; granular excipients and lubricant were blended uniformly.
Tabletting
Omeprazole coated pellets were compressed into tablets with a thickness of 6.5 mm using 11 × 8.5 mm punch. Compression of tablets in an industrial type machine was performed using 24 station rotary tablet press (Cadmach, Mumbai). The compositions of tablet formulations were shown in Table 2.
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| Pellets | 203.34 | 230.11 | 216.58 | 230.11 | 216.58 | 230.11 | 216.58 | 230.11 |
| Prosol HD 90 + prosol SMCC | 368.19 | 391.19 | 368.19 | 391.19 | 368.19 | 391.19 | 368.19 | 391.19 |
| Crosspovidone XL | 20.33 | 23.00 | 21.66 | 23.00 | 21.66 | 23.00 | 21.66 | 23.00 |
| Sodium stearyl fumurate | 40.67 | 46.02 | 43.32 | 46.02 | 43.32 | 46.02 | 43.32 | 46.02 |
| Total wt. of tablet | 650.02 | 690.33 | 650.00 | 690.33 | 650.00 | 690.33 | 650.00 | 690.33 |
Table 2: Prototype formulations: Enteric coated pellets containing 40 mg omeprazole per unit tablet
Scanning electron microscope
The shape and surface morphology of the pellets before and after compression were examined with a scanning electron microscope (JOEL–JFC 5300). The pellets were mounted on adhesion stuff and then coated with 30 nm layer of gold platinum under vacuum using an ion sputter coater (JOEL-JFC 1000E). The coated specimens were observed under the SEM at 15 kv and SEM micrographs were taken.
Differential scanning calorimetry
Thermal characterization of pure drugs and tablet formulation were performed with Mettler Toledo, USA. About 10 mg of sample was placed in sealed aluminium pan. The equipment was calibrated with indium. The samples were scanned at 20°C/min from 50-400°C.
X-ray diffraction
Crystallinity of the pure drug, drug in final formulation, placebo was determined by using Bruker AXS D8 Advance with copper target. The conditions were 40 kv voltage, 30 mA current at room temperature. The samples were loaded on to diffractometer and scanned over a range of 2-theta values from 3° to 60° at a scan rate of 0.05°/min.
In vitro drug release
The in vitro drug release study of final formulations were performed using USP type II dissolution testing apparatus at 100 rpm paddle speed and temperature of bath was maintained at 37.5°C. Dissolution testing of all formulations was performed in triplicate.
A gastro resistant study was performed by placing the tablets into dissolution vessel containing 900 ml of simulated gastric fluid. The test was run in gastric fluid for 2 h. At the end of 2 h, the gastric fluid was discarded and replaced with 900 ml of phosphate buffer PH 6.8. Dissolution testing was run in phosphate buffer for another 1 h for omeprazole release. 5 ml of samples were collected from each vessel at 5, 10, 15, 30, 45, 60 min. The samples were then filtered through membrane filters (0.45 μm) and measured using UV spectrophotometer at 305 nm. The drug release was determined using an appropriate standard curve. The dissolution profiles of formulations were presented as percent drug release vs. time curves.
Results and Discussion
Micromeritics properties of excipients
The powder blends were evaluated for flow properties such as bulk density, tapped density, compressibility index, Hauser ratio. Batch T3 (70% prosolv HD90, 30% prosolv SMCC90) shows the good flow, compressibility and less weight variation. It proves that blends of different combinations of excipients possess comparable compressibility and flow properties. The results were shown in Table 3.
| Batch code | Composition | Bulk density | Tapped density | Compressibility index | Hausner ratio |
|---|---|---|---|---|---|
| T1 | T1(100%MCC) | 0.581 | 0.724 | 19.75 | 1.24 |
| T2 | T2(50% prosolv HD90, 50%prosolv SMCC90) | 0.585 | 0.693 | 15.58 | 1.18 |
| T3 | T3(70% prosolvHD90, 30%prosolv SMCC90) | 0.64 | 0.72 | 12.82 | 1.15 |
Table 3: Micromeretic properties of excipient blend with different compositions of excipients
Tablet properties
The eight tablet formulations containing enteric coated pellets equivalent to 40 mg of omeprazole were tested for their mechanical properties i.e., crushing strength, friability and disintegration time. The results were an average of six tablets from each batch. The hardness of tablets was found to be 8-10 kg/cm2. Friability below 1% was an indication of good mechanical resistance of tablets. Table 4 proved that all batches were found to be within the limits.
| Tablet properties | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 |
|---|---|---|---|---|---|---|---|---|
| Crushing strength (kp) | 8.0 | 8.6 | 9.4 | 10.0 | 10.5 | 10.8 | 10.5 | 11.0 |
| Friability (%) | 0.98 | 0.92 | 0.83 | 0.75 | 0.63 | 0.56 | 0.59 | 0.52 |
| Disintegration time (min) | 1:45 | 1:52 | 1:49 | 1:50 | 1:55 | 2:00 | 2:05 | 2:15 |
Table 4: Mechanical properties of tablets.
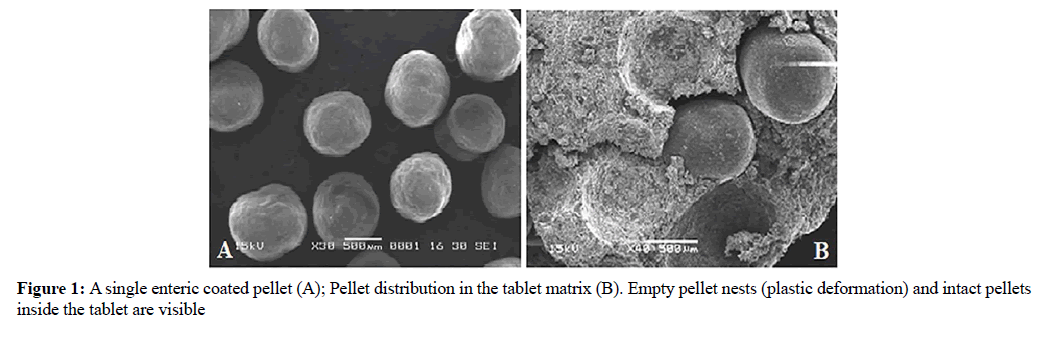
Morphology of pellets
The morphological characterization of pellets would help to correlate the particle size and surface characteristics with other determined properties. The pellets obtained after coatings were spherical in shape and had relatively smooth surface without any pores. The SEM photos were taken by breaking a tablet in half. Figure 1a shows a single enteric-coated pellet; Figure 1b shows a cross-section of a tablet (F4) containing individual pellets scattered inside a supporting matrix. One can observe the matrix deformation corresponding to a single pellet and there was no visible damage to the pellets. However, only damage to the enteric coating occurred on the surface of the tablets. Granulation of the excipients to match the pellet size had a crucial role in absorbing the compression pressure. The prosolv matrix showed strong plastic deformation and all the pellets inside a tablet maintained their shape with no significant change in their surface properties.
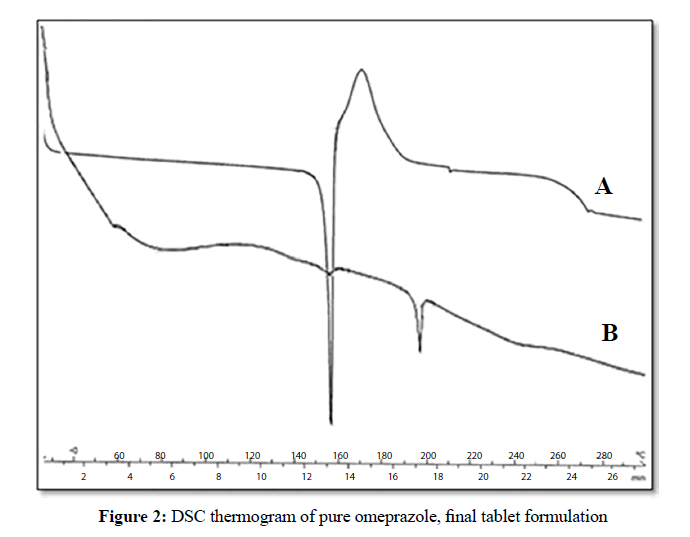
Thermal characteristics of tablets
DSC thermographs of free omeprazole and final tablet formulation (Figure 2) were made in the attempt to detect the occurrence of interactions, if any between the drug and polymer within formulation. The melting endotherm of pure omeprazole was found to be 157°C. The thermograph of tablet formulation was similar to that of omeprazole and shows a detectable endotherm corresponding to the melting point of free drug between 155-160°C. The results suggest that the drug was at molecular level at polymer melting temperature and the drug maintained its characteristics in tablet formulation.
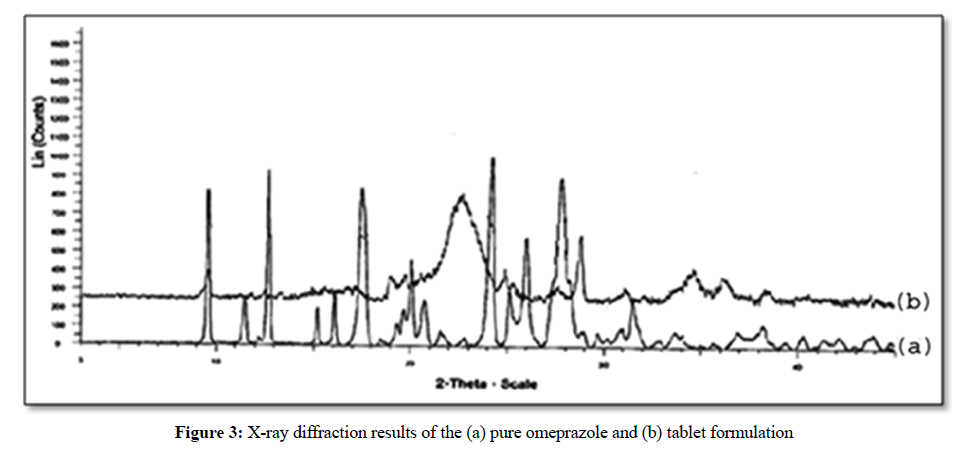
X-ray diffraction
The crystallinity of prepared tablets was studied by X-ray diffraction. Figure 3a shows the X-RD pattern for omeprazole. The diffraction peaks were at 9.54°, 12.482°, 18.991°, 22.435°, 28.97° coincides with standard pattern of omeprazole which indicates that the tablet formulation (Figure 3b) did not change the typical spectra of omeprazole and the diffraction peaks were similar to that of omeprazole. From the X-ray diffraction it can be concluded that the drug in tablet formulation existed in crystalline form on the sugar sphere after fabrication of formulation.
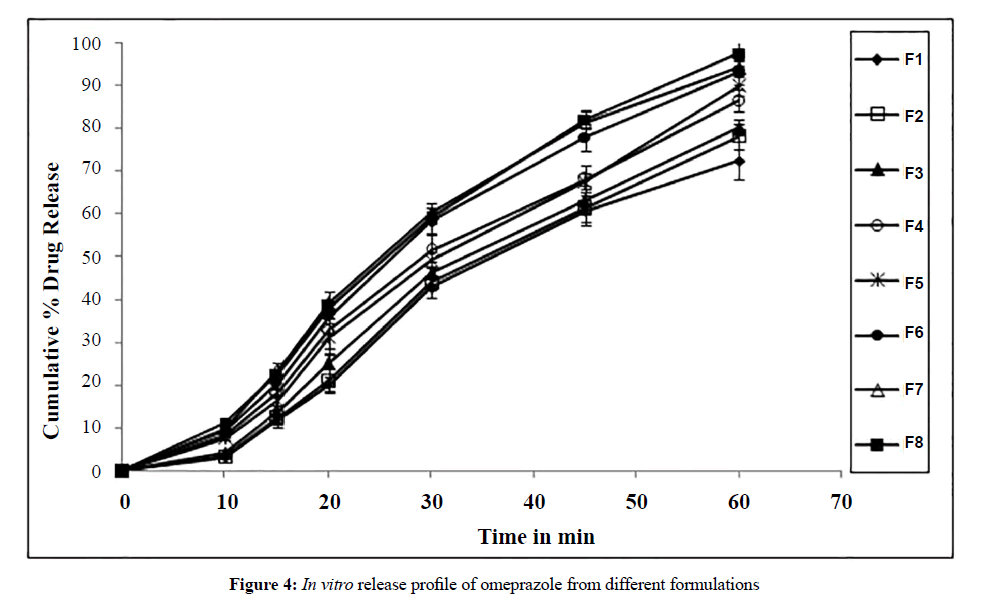
In vitro release studies
The criterion of modified release system is its gastro intestinal resistance against gastric environment. It is necessary that less than 10% of drug degradation would occur after 2 hrs in stimulated GI fluids. All formulations complied with the condition. All formulations (F1-F8) disintegrate freely in less than 3 min. The formulations F1, F2 containing #18/20 sugar spheres exhibit 72% and 78% release and the formulations F3, F4 containing #20/25 sugar spheres showed 80% and 86% release respectively as they are not resistant to acid due large size of pellet and more damage to coating during compression. The formulations F5, F6 comprising #30/35 sugar spheres showed 90% and 93% and the formulations F7, F8 comprising #40/60 sugar spheres showed 94%, 97% release respectively. These batches shows comparatively better release than above due to more acid resistance and less damage to the coating. Among all the formulations F7, F8 shows less acid release and maximum release of omeprazole in buffer. The release behaviour of formulations F1-F8 was shown in Figure 4.
The size of pellets influences the drug release. The higher the size of sugar spheres higher will be the smash up to the coating and less resistance to gastric environment than the small spheres [12]. The quantity of polymer coating has its own role in protecting polymer film integrity during compression. A thicker coating can withstand damage better than a thinner one [22]. Formulations F1, F3, F5 and F7 with 50% Eudragit coating has less elasticity and unable to withstand the compression force, whereas formulations F2, F4, F6 and F8 with 70% Eudragit coating offer better resistance to compaction forces. The release was shown in Figure 4.
The size of pellet has influence on release of drug from compressed tablet [23,24]. Formulation F8 containing #40/60 sugar spheres with 70% Eudragit coating yields maximum release of 97% within 1 hr, which has concluded that among all the batches F8 was found to be the best formulation. In conclusion formulation F8 containing #40/60 sugar spheres with 70% Eudragit coating enhances the release from 70% to 96% within 1 hr.
Conclusion
The work is optimized for the size of sugar spheres suitable for compression of enteric coated omeprazole pellets in to tablets. The pellets were prepared in a layering process by wurster technology using different sizes of sugar spheres (#18/20, 20/25, 30/35, 40/60) as inert core. Drug loading on the sugar spheres was done by using HPMC as binder. The percentage build-up for enteric coating was optimized by acid resistance of pellets. The studies proved has the pellet size decreases percentage build-up should be more to withstand the acid exposure.
Enteric coated pellets were evaluated for gastric resistance test and in vitro drug release study. All the formulations revealed satisfactory results. From the release profiles it had been concluded that the size of the pellets influence the compaction properties and in vitro release. The increase in the size of sugar spheres resulted in more damage to the coating and less resistance to gastric environment than the small spheres and hence the small pellets were best suited for compression of multiple unit particulate systems of Omeprazole Among all the batches, formulation comprising sugar spheres #40/60 exhibits maximum in vitro release due to more acid resistance and less damage to the coating.
References
- Gisbert, JP., et al., Digestive Diseases and Sciences, 2002. 47(1): p. 471-488.
- Ghebre, I., Multiparticulate Oral Drug Delivery, New York, Marcel Dekker, 1994.
- Bechgward, H., Nielson, GH., Drug Development and Industrial Pharmacy, 1978. 4(1): p. 53-67.
- Jalal, IM., Malinowski, HJ., Smith WE., Journal of Pharmaceutical Sciences, 1972. 61(3): p. 1466-1790.
- https://www.interphex.com/Newsroom/Press-Releases/
- Watanalumerd, P., Christensen, JM., Ayres, JW., Pharmaceutical Development and Technology, 2007. 12(2): p. 193-202.
- Bodmeier, R., European Journal of Pharmaceutics and Biopharmaceutics, 1997. 43(5): p. 1-8.
- Celik, M., Ghebre, S., Marcel Dekker, NY, USA, 1994.
- Jaslin, M., Turakka, L., Puumalainen, P., Indian Journal of Pharmaceutical Sciences, 1980. 42(3): p. 829-832.
- Gazaerly, EI., Rakkanka, V., Ayres, JW., Pharmaceutical Development and Technology, 2004. 9(2): p. 181-88.
- Haslam, JL., et al., International Journal of Pharmaceutics, 1998. 173(9): p. 233-242.
- Ragnarsson, G., et al., Drug Development and Industrial Pharmacy, 1987. 13(9): p. 1495-1509.
- Aulton, ME., Dyer, AM., Khan, KA., Drug Development and Industrial Pharmacy, 1994. 20(3): p. 3069-3104.
- Chang, RK., Rudnic, EM., International Journal of Pharmaceutics, 1991. 70(2): p. 261-270.
- Bodmeter, R., Paeratakul, O., Pharmaceutical Research, 1994. 11(6): p. 882-888.
- Nickalson, F., Alder, G., European Journal of Pharmaceutical Sciences, 1999. 9(2): p. 57-65.
- Hab, RB., James, WA., European Journal of Pharmaceutics and Biopharmaceutics, 2008. 69(3): p. 977-985.
- https://www.diva-portal.org/smash/get/diva2:162716/FULLTEXT01.pdf
- https://ei.is.tuebingen.mpg.de/publications/jarvis2015science
- Pilbrant, A., Journal of Gastroenterology, 1985. 20(1): p. 113-119.
- Stroyer, A., Meginity, JW., Leopold, CS., Journal of Pharmaceutical Sciences, 2006; 95(6): p. 1342-1353.
- Flament, MP., et al., Pharmaceutical Technology Europe, 1994. 6(4): p. 19-25.
- Pinto, JF., Podczeck, F., Newton, JM., Chemical and Pharmaceutical Bulletin, 1997. 17(2): p. 171-180.
- Zimm, KR., Krumme, M., Schmidt, PC., Sciences Techniques et Pratiques Pharma Sciences, 2000. 10(2): 327-334.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences