ISSN : 2393-8862
American Journal of Pharmacology and Pharmacotherapeutics
Drug Compatibility and Distribution Assessments of a Bupivacaine-Polymer Formulation Measured Following Site-Directed Injection in Mice
Ickowicz DE1, Houri-Haddad Y2, Domb AJ3, Golovanevski L4, Weiniger CF5*
1Institute for Drug Research, School of Pharmacy, The Hebrew University of Jerusalem, Israel
2Department of Prosthodontics, Faculty of Dental Medicine, Hadassah, Hebrew University Medical Center, Ein Kerem, Jerusalem, Israel
3Institute for Drug Research, School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem, Ein Kerem, Jerusalem, Israel
4Department of Anaesthesiology and Critical Care Medicine, Hadassah Hebrew University Medical Centre, Isreal
5Department of Anaesthesiology and Critical Care Medicine, Faculty of Medicine, Hebrew University of Jerusalem, Jerusalem, Israel
Abstract
Background: A long-acting local anaesthetic agent would enable superior post-operative analgesia. Our slow-release bupivacaine-polymer formulation comprising 15% bupivacaine loaded into a poly (DL-lactic acid co castor oil 3:7), DL-LA:CO was effective for up to 96 hours. In this study we determined in vivo drug levels of the formulation from plasma and tissue assay and we tested the feasibility of drug level assessments from an exudate sample collected in a novel device near the injection-site.
Methods: Prior to formulation injection, a titanium cylinder dual-open-ended chamber was implanted in the dorso-lumber region of female ICR mice, to be used as a biological compartment. Following a healing period, the bupivacaine-polymer formulation was injected close to the sciatic nerve. Formulation assay samples were collected over one-week post-injection; exudate was drawn from the chamber in the anaesthetised animal; and blood and tissue samples from the sacrificed animal. Bupivacaine distribution in the exudate and plasma samples was determined by liquid chromatography–mass spectrometry developed for these experiments, and in tissue samples by high -performance liquid chromatography.
Results: Bupivacaine was detected during the first 24h following formulation injection in all three samples (exudate, plasma and tissue), and decreased to almost undetectable levels by day 7 post-injection.
Conclusion: Bupivacaine drug assay measured from an exudate sample may be applicable in addition or as a replacement for standard plasma and tissue assay in studies of drug pharmacokinetic distribution. The high sensitivity and simplicity of the metallic chamber implant to detect drug concentration and exudate analysis was confirmed in sever hepatic impairment.
Keywords
Biodegradable polymer, Bupivacaine, Castor oil, Lactic acid, Pharmacokinetics.
Abbrevations
ACN: Acetonitrile; CXP: Cell Exit Potentials; HPLC: High-Performance Liquid Chromatography; DL-LA:CO: Poly(DL-Lactic Acid Co-Castor Oil 3:7); DDW: Double Distilled Water; ESI: Electrospray Ionisation; IPA: Isopropanol; MRM: Multiple Reaction Monitoring; MP: Mobile Phase; MS: Mass Spectrometry; LOD: Limit of Detection; LOQ: Limit of Quantification; IS: Internal Standard; LC/MS: Liquid Chromatography–Mass Spectrometry
Introduction
Long-acting local anesthetic agents can provide superior analgesia for pain management is important, since efficacy of these drugs in a clinical setting is limited by short duration of action [1]. Long-acting site-directed local anesthetics can prolong the duration of pain relief; usually achieved through continuous catheter drug infusion. Recent advances have led to clinical availability of long-acting local anesthetic agents for directly placed site-directed application [2,3].
Alternative options for site-directed long-acting local anesthetic agents are in development. We previously reported up to 96 hours of analgesia following site-directed injection application of a slow release of a poly (DL-lactic acid co castor oil 3:7), DL-LA:CO, formulation loaded with 15% bupivacaine, a local anesthetic agent [4].
Assessment of local toxic effects is an essential step during drug development [5]. The standard measurement of toxic drug effects is through plasma and tissue sampling that involves sacrificing the animal to obtain the plasma and tissue samples. Such drug assays can be performed in the supernatant by High-performance liquid chromatography (HPLC); an accurate and rapid method for the measurements required for regulatory approval [6]. Bupivacaine concentration in human plasma has been determined using HPLC [7-10] and mass spectrometry (MS) [6,11].
In the current work, we developed a novel method of in vivo tissue sampling through an exudate sample obtained from the injection site, in addition to samples obtained for bupivacaine drug assays using standard measurement from tissue and plasma, in order to determine drug concentration and compare the drug distribution in these three samples after injection of the bupivacaine-polymer formulation.
Materials and Methods
The study received approval from the Ethics Committee of the Hebrew University Hadassah Medical School (OPRR-A01-5011) for performance of animal studies (MD-11-12672-4). Experiments were performed following the ARRIVE guidelines. Female ICR mice (25 g-35 g) were housed in the Specific Pathogen Free laboratories of the Hebrew University, ten per cage with free access to food and water. The animal room was light cycled (12 h light, 12 h dark), and the temperature was 22°C.
The exudate samples
A subcutaneous inert titanium chamber (1.5 cm) was used as a biological compartment, and its exudate content sampled at specified time intervals without need to sacrifice the animals. Titanium is highly biocompatibility thus local inflammatory reactions were avoided (Figure 1). This selected model was developed to examine, the host response and the effect of anti-inflammatory drugs on the immune response [12,13]. The titanium chamber was implanted in the subcutaneous tissues in the left dorso-lumbar region of the mice following anaesthesia with an intraperitoneal injection of ketamine: xylazine (80: 20 mg/Kg) [12,13]. The chamber was easily palpated under the skin and permitted easy needle access to withdraw the exudate that gathered as it was open from both sides [12,14]. The first week following chamber implantation was a healing period, as the chamber became covered by loose connective tissue, rich in blood vessels. During this healing period, animals were weighed and followed daily for behavioural changes.
Formulation raw materials
DL-lactide Purasorb, Purac Amsterdam; Tin(II) 2-ethylhexanoate, (Sn(Oct)2), Sigma Israel; castor oil, Tamar Pharm Israel; bupivacaine HCl USP 26 Eurotrade, Commerce, S.L; Liquid chromatography–mass spectrometry (LC/MS) grade methanol and water were purchased from Biolab Ltd. Jerusalem.
Formulation preparation
Poly(DL: lactic acid co castor oil) 3:7 designed as p(DLLA-CO) 3:7 with Mn 2200 and Mw 2300 Da was synthesised from castor oil and DL-lactide as previously described. [15] Bupivacaine free base was prepared from bupivacaine hydrochloride by alkaline precipitation and filtration as previously described [15] . Bupivacaine free base (15%, w/w) was reduced to 0.25 mm particle size by sieving the powder through a 60-mesh sieve and the powder was incorporated by mixing into the liquid polymer at room temperature to produce a viscous injectable liquid.
Formulation injection
Following the one-week healing period, mice were divided randomly into five groups (n= 28) and bupivacaine-polymer 15% formulation (0.1 ml) was injected via a needle of 22G diameter directly close to the left sciatic nerve using a nerve stimulator (StimuplexR B. Braun Melsungen AG, Germany) at 0.2 mA and 1 Hz to identify the sciatic nerve, under ketamine: xylazine anaesthesia [16].
Animal behaviour
Animal behaviour was monitored followed for signs of toxicity such as weight loss, agitation, malaise, aggression over the 7 days study period.
Sample collection
Three samples were retrieved: plasma, tissue at the injection site and chamber exudate. Samples were obtained at days, 1,2,3,4 and 7 following the bupivacaine-polymer injection.
The exudate sample was drawn from the live non-anaesthetized animal using a 24 G needle that entered directly into an open end of the subcutaneous chamber. The exudate was withdrawn into a tuberculin syringe, typically 0.1 μl-03 μl.
The tissue and plasma samples were taken, following euthanasia (pentobarbital 100 mg/Kg followed by cervical dislocation). Blood samples were drawn from the eye socket, followed immediately by protein precipitation to obtain plasma. Tissue was retrieved from around the sciatic nerve, cleaned, the skin removed, and the sample homogenized.
Analytical methods
For plasma, exudate and tissue were selected according to preliminary tests and expected drug concentrations. LC/MS was selected for plasma and exudate and HPLC for tissue samples. Sample preparation was adapted according to sample size/volume collected and to measurement method selected.
Instrumentation
For plasma and exudates, chromatographic separation of bupivacaine and the internal standard, lidocaine (IS), were performed using a Shimadzu (Kyoto, Japan) UHPLC System, series Nexera, consisting of a CBM-20A LITE controller, two LC-30AD pumps, a Prominence DGU-20A5R degasser, a SIL-30AC autosampler and a CTO-20AC column oven. A C18, 2.6 μm (100 Å pore size, 50 × 2.1 mm) column, Kinetex (Phenomenex, USA) was protected by a SecurityGuard™ (Phenomenex, USA) ULTRA cartridges (C18, 2 × 2.1 mm). MS System: AB SCIEX Triple Quad™ 5500, positive mode. Bupivacaine tissue concentrations were determined using HPLC with a C18 reverse phase column Luna (5 μm, 250 × 4.6 mm).
Data acquisition and analysis of the LC/MS system were performed on a Dell OptiPlex 960 computer with Analyst 1.6 software, AB Sciex. Quantitative calibration (0-30 ng/ml bupivacaine) was performed before every batch of samples. The chromatographic separation was achieved using a gradient method with a mobile phase (MP): [A= 0.1% FA in water, B=0.1% FA in Acetonitrile (ACN)] at a flow rate of 0.45 ml/min over a total run time of 8.5 min.
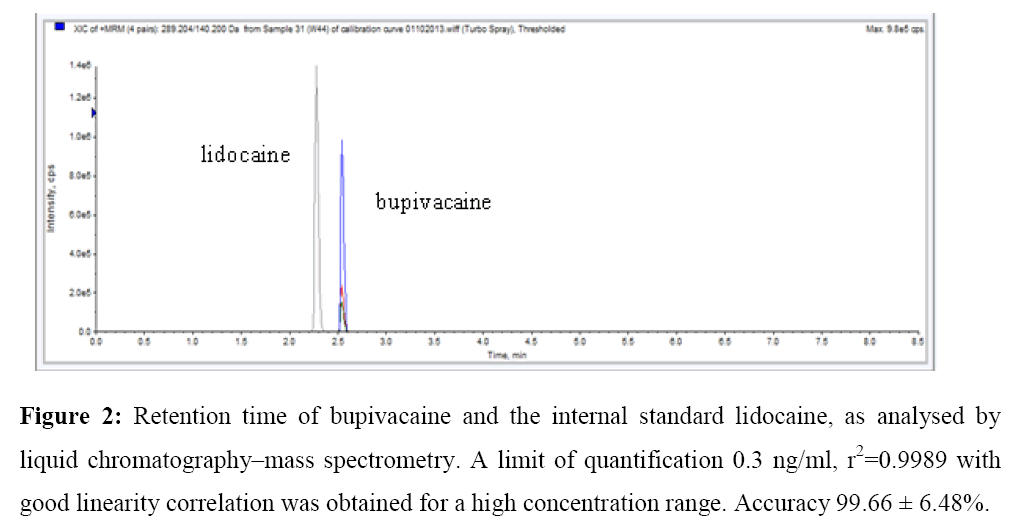
Linearity range is 0.3-30 ng/ml. Chromatographic oven temperature was set at 40°C. Injection volume: 1 μl. Retention time 2.3 minutes, lidocaine; 2.56 minutes bupivacaine (Figure 2). The chromatographic data were automatically processed for peak-height ratios of the analytes to the IS and fitted to a weighted linear regression. No significant interfering peaks were found at the retention time of bupivacaine and the IS. A limit of quantification (LOQ) 0.3 ng/ml r2=0.9989 with good linearity correlation, was obtained for a high concentration range, accuracy 99.66 ± 6.48%.
Preparation of plasma samples
For each evaluation point, 50 μl of plasma were mixed with 150 μl of water, 50 μl of IS solution, and 50 μl of MP. Protein precipitation was obtained by addition of 600 μl of ACN, followed by centrifugation at 12000 rpm for 10 min at 4°C. The resultant clear supernatant was filtered through a 0.22 μm filter and analysed by LC/MS. For day 1 and 2, plasma samples were dissolved 1:4 with water. The diluted solution was processed as described above.
Preparation of exudate samples
Exudates samples were evaluated in a similar manner as for plasma. For each evaluation time-point 25 μl of exudates were mixed with 75 μl of water, 25 μl of IS solution and 25 μl of MP. Protein precipitation was obtained by addition of 300 μl of ACN and centrifuge at 12000 rpm for 10 min. Supernatant clear solution was filtered and analysed by LC/MS.
Standard solutions and calibration curves for plasma and exudates samples
Stock solutions of bupivacaine were prepared in formic acid (0.1%) 50%: ACN 50% and stored at 4°C. Lidocaine stock solution was prepared in water to a final concentration of 2.5 ug/ml and used as IS with a final concentration of 138 ng/ml. Each concentration was prepared in duplicate. 100 μl of plasma were diluted 1:2 with double distilled water (DDW) and 50 μl of the bupivacaine solution and 50 μl of lidocaine 2.5 μl/ml were added. Drugs were extracted with 600 μl of ACN, centrifuged 10 minutes at 12000 rpm, and filtered through a 0.22 μm filter. For recovery calculation, 200 μl of water was used.
Bupivacaine was detected using positive ion mode using electrospray ionisation (ESI) and multiple reaction monitoring (MRM) mode of acquisition. Optimal detection conditions were determined by constant infusion of a 100 ng/ml solution of Bupivacaine in 9:1 water: MeOH using the integrated syringe pump (5 μL/min). The molecular ion of [Bupivacaine+H]+ (m/z 289.2) was selected in the first mass analyser and fragmented in the collision cell, followed by detection of the products of fragmentation in the second mass analyser. One transition was monitored for bupivacaine: m/z 289.2 → 140.2. Table 1 presents the collision energies and collision cell exit potentials (CXP) for bupivacaine and lidocaine transitions.
| MRM transitions | Q1 (m/z) | Q3 (m/z) | CXP (V) collision cell exit potential | CE (eV) collision energy |
|---|---|---|---|---|
| Lidocaine | 234.9 | 86.3 | 6 | 45 |
| Bupivacaine | 289.2 | 140.2 | 12 | 29 |
Q1: Quadrupole 1; m/z: Mass to charge ratio; Q3: Quadrupole 3; CXP: Collision cell exit potential; CE: Collision energy; V: Volts; eV: Electron volts
Table 1: Multiple reaction monitoring (MRM) transitions and parameters for bupivacaine and lidocaine (IS).
Preparation of tissue samples
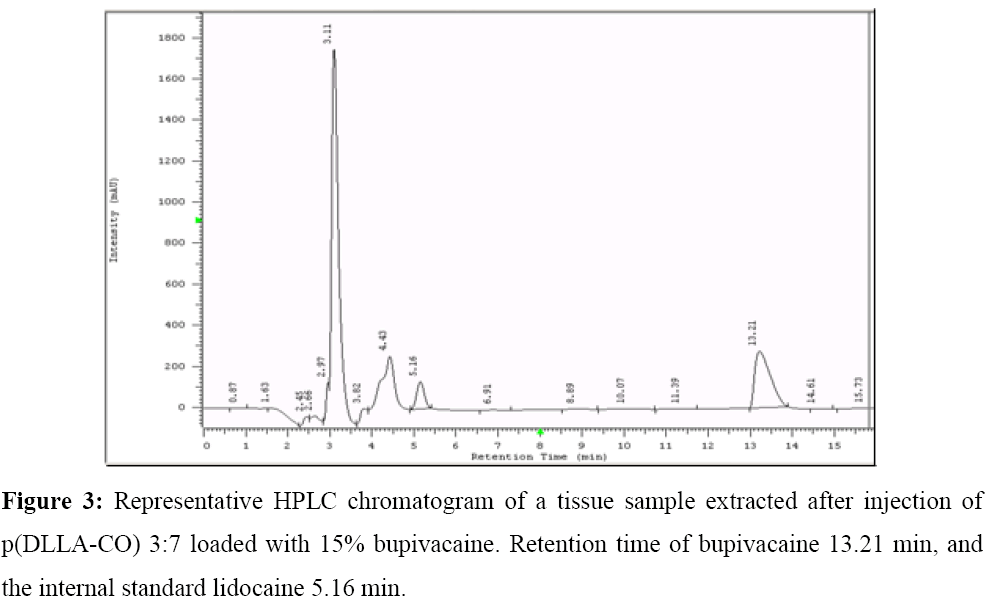
All tissue samples were suspended in buffer phosphate 0.5% (120 mg/ml) vortexed for 10 min and homogenised. To 500 μl of the homogenate, 100 μl of IS solution were added. Protein precipitation was obtained by addition of 4.2 ml of Isopropanol (IPA), followed by centrifugation at 12000 rpm for 40 min at 4°C. 2.4 ml of the resultant clear supernatant was evaporated to dryness and reconstituted in 200 μl of buffer phosphate 0.01M pH 3 and filtered through a 0.22 μm filter. Samples were analysed by HPLC using a Luna 5 μm C18 column. A mixture of 75% buffer phosphate 0.01M pH 3: 25% ACN at a flow rate of 1 ml/min was used as eluent with UV detection at 210 nm (injection volume 50 μl, running time 16 minutes). Retention times were 5.15 minutes lidocaine and 13.2 minutes bupivacaine (Figure 3).
A linear calibration plot for assay method was obtained over the calibration range, with a correlation coefficient of 0.9891. The results showed an excellent correlation between the peak area and concentration of the analyte. LOQ was determined as signal-to-noise ratio of 10:117. Calibration curves for bupivacaine in release medium were obtained by programmed injection of different aliquots (50 μl) of a standard solution in concentrations from 0-160 ug/ml.
Standard solutions and calibration curves for tissue samples
Stock solutions of bupivacaine were prepared in buffer phosphate 0.5% and stored at 4°C. Lidocaine stock was prepared in water (250 ug/ml) and used as IS. Each concentration was prepared in duplicate. 300 mg of tissue was suspended in 1 ml of bupivacaine solution (at different concentrations) and the volume completed to 2.5 ml with buffer phosphate pH 3, and vortexed for 10 minutes. Later, the tissue was homogenised with a tissue homogeniser at 12000 rpm, and then 100 μl of lidocaine were added to 500 μl of the homogenate. Drugs were extracted with 4.2 ml of IPA, homogenised again, centrifuged 40 minutes at 12000 rpm at 4°C and filtered through a 0.22 μm filter. Of the supernatant, 2.4 ml were evaporated to dryness and re-suspended in 200 μl of buffer phosphate pH 3. DDW (300 μl) was used to calculate recovery. MP: 75% Buffer Phosphate 0.01M pH 3: 25% ACN (injection volume 50 μL, run time 16 minutes). Drug concentrations were calculated according to the ratio of IS: bupivacaine and recovery was calculated according to tissue:water concentrations. The limit of detection (LOD) was calculated as signal-to-noise ratio of 3:1, and the LOQ was determined as signal-to-noise ratio of 10:1 Recovery 85 ± 0.09%.
Results
Following implantation of the titanium subcutaneous chamber, all mice recovered well throughout the 7 days experimental period. Animal weight increased normally during the healing period after chamber placement, and animals did not demonstrate signs of toxicity.
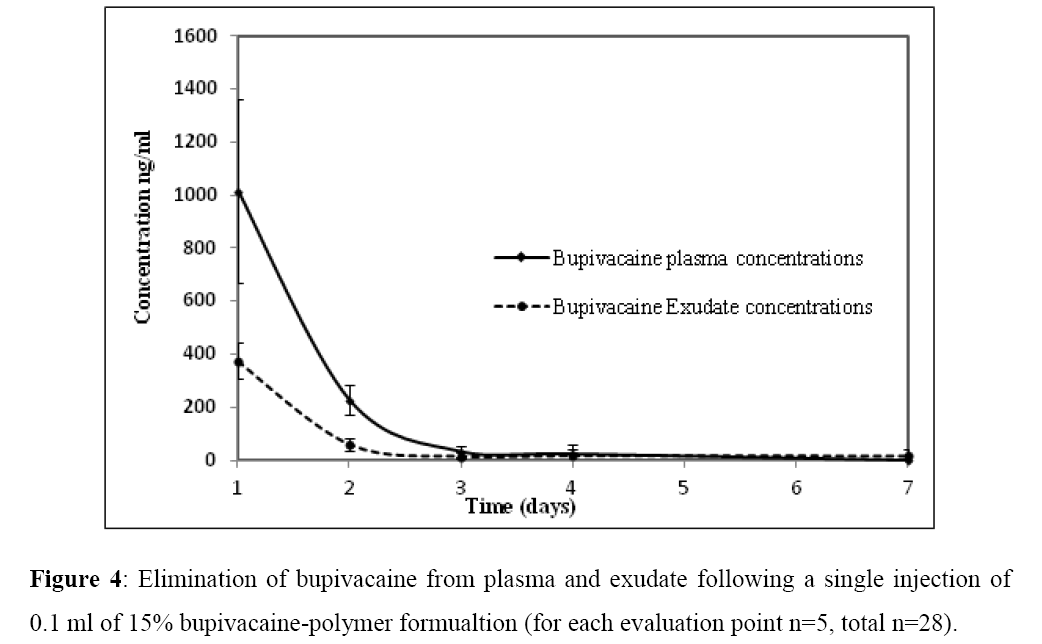
The maximal concentrations of drug observed in plasma, tissue and exudate samples were observed 24 h following site-directed injection of the bupivacaine-polymer formulation. The drug concentrations followed a similar distribution pattern in both plasma and exudate samples with highest concentrations observed during the first 24 h. Bupivacaine concentrations fell during the experimental period (Figure 4). Concentrations of bupivacaine in the exudate samples at each evaluation point were about half of the drug concentration measured in plasma.
The bupivacaine concentrations measured in the extracted tissue samples revealed maximal concentrations 24 h following the injection, as shown for plasma and exudate. At this evaluation point, the maximum concentration of bupivacaine measured in tissue from the injection site was 7.03 ± 2.75 ug/g. After 48h, bupivacaine concentrations in tissue from the injection site decreased to 54.04 ± 11.28 ng/g. By 72 hours following injection the remaining concentrations at the injection site were 15.71 ± 8.2 ng/g. After this time, the concentrations were below the limit of detection. Drug concentrations were almost undetectable by 72 h following injection.
Comparison of the non-injected right leg did not reveal measured drug concentration at any evaluation point in the tissue samples. The concentrations of drug found in tissue were higher than those found in plasma and exudates.
Discussion
In this investigation, we measured a bupivacaine-polymer formulation assay in vivo from an exudate sample collected from the injection site in addition to standard plasma and tissue assay. We observed maximal concentrations of drug samples 24h following site-directed injection. Toxicity of bupivacaine has been associated with drug concentrations in plasma and the need for animal models to study this effect is well reported [18,19].
Dal Bo et al. [20] described a similar method to analyse lidocaine concentrations in plasma, using bupivacaine as IS. Lidocaine concentrations of the interstitial fluid near the sciatic nerve were evaluated relative to tissue drug concentration after implantation of extended release formulation [21]. Our results suggest that there was no interference from the plasma matrix, and support determination of biological fluid assays using these methods. The relationship between exudate drug concentrations and plasma concentrations confirms that exudate sampling may be adapted in future studies for pharmacokinetic analysis.
In our investigation, despite the longer half-life of the bupivacaine-polymer (14 h) relative to commercial plain bupivacaine (3 h), the clearance from the injection site was faster than expected. This may be related to the volume of tissue analysed. The site-directed injection was guided using nerve stimulation to locate the nerve; however, this apparatus does not allow precise identification of this tissue for sample collection. The injection site was marked in order to specify the zone for tissue sample collection, however if smaller focused tissues samples could be collected, potentially higher drug concentrations could be identified at the site of injection. Alternatively, redistribution of the drug in the area surrounding application may explain this finding.
The use of a comparison leg without measured drug concentration at any evaluation point in the tissue samples could be used to understand drug redistribution at the injection site.
Our finding that tissue drug concentrations were higher than those found in plasma and exudates, may addressed through further dilution of the samples in order to achieve suitable concentrations for LC/MS and to avoid saturation of the column. Using one single measurement method can circumvent the need for two different columns, one for HPLC and one for LC/MS, and reduce differences when comparing results.
One limitation of selecting LC/MS as the best analytical method in our study was the requirement for different dilutions for each sample type to quantify the desired concentrations while avoiding saturation of the measurement system. The measurement technique utilised may produce different results: HPLC has a different sensitivity than mass spectrometry. LC/MS is a very sensitive measuring technique and this property facilitates detection of very low drug concentrations, thus if low drug concentrations are expected in the site to be tested, LC/MS is a suitable method. Conversely, use of LC/MS to detect high concentrations reduces sensitivity of the measure as the column system can be easily saturated. HPLC is a simple less costly technique that is more suited for higher concentrations. As we expected to find high concentrations of drug in the tissue (>100 ug/g) we selected HPLC to measure tissue samples. Our results suggest that LC/MS was a suitable technique for all our samples given the lower concentrations seen in the tissues. Use of the same method to measure all samples will enable higher sensitivity and avoid the need to develop different methods that each require calibration. Use of radioactive-labelling of the drug in the formulation may enable further understanding of the drug journey in exudate, tissues and plasma following injection of the formulation [22,23]. We limited the sample size as animals were euthanised at each evaluation point in order to obtain plasma and tissue samples that could be compared to the exudates. Finally, we measured the total concentration of bupivacaine in plasma rather than free concentration.
Conclusion
The drug assays we performed in the current study suggest a novel method to measure drug distribution in exudate samples in the live animal, corresponding to measures of plasma and tissue samples following site-directed injection. This may enable a simple measurement tool that could be used for pharmacokinetic analysis in clinical studies, without requiring sacrifice to retrieve the tissue samples. We were able to detect low drug concentrations due to the very low sensitivity (LOD 0.3 ng/ml). We demonstrated that drug distribution was comparable following the release from the injected site. The subcutaneous chamber with exudate sampling could be used as a measurement tool for pharmacokinetic drug analysis in the live animal.
Declaration of Interests
The authors have no competing interests to declare.
Funding
This research was supported by the Legacy Heritage Science Initiative Program of the Israel Science Foundation (1637⁄10), in part by grant no. 300000-4889 from the Chief Scientist Office of the Ministry of Health, Israel, by a physician research grant from the Hadassah-Medical-Organization and by Eizenberg's grant for young researchers from Hadassah Medical Center, Israel. This work was not funded by: National Institutes of Health (NIH), Howard Hughes Medical Institute (HHMI), Medical Research Council (MRC), and/or Wellcome Trust.
References
- Pedersen JL., Lillesø J., Hammer NA., et al. Bupivacaine in microcapsules prolongs analgesia after subcutaneous infiltration in humans: A dose-finding study. Anesthesia Analgesia2004;99:912-918.
- Golf M., Daniels S., Onel E., A phase 3, randomized, placebo-controlled trial of DepoFoam® bupivacaine (extended-release bupivacaine local analgesic) in bunionectomy. Adv Therapy2011;28:776-788.
- Gorfine SR., Onel E., Patou G., et al. Bupivacaine extended-release liposome injection for prolonged postsurgical analgesia in patients undergoing hemorrhoidectomy: A multicenter, randomized, double-blind, placebo-controlled trial. Dis Colon Rectum 2011;54:1552-1559.
- Ickowicz DE., Golovanevski L., DombAJ., et al. Extended duration local anaesthetic agent in a rat paw model. Int J Pharm 2014;468:152-157.
- ICH.,Q2B: Validation of analytical procedures and methodology. 1996.
- SalamaNN., Wang S., Quantitative mass spectrometric analysis of ropivacaine and bupivacaine in authentic, pharmaceutical and spiked human plasma without chromatographic separation. Anal ChemInsight 2008;4:11-19.
- Qin WW., Jiao Z., Zhong MK., et al. Simultaneous determination of procaine, lidocaine, ropivacaine, tetracaine and bupivacaine in human plasma by high-performance liquid chromatography. J Chromatograph 2010;878:1185-1189.
- Lindberg RLP., PihlajamäkiKK., High-performance liquid chromatographic determination of bupivacaine in human serum. J Chromatograph: Biomed SciAppl 1984;309:369-374.
- Ha HR., Funk B., Gerber HR., et al. Determination of Bupivacaine in Plasma by High-Performance Liquid Chromatography. Anaesthesia Analgesia. 1984;63:448-450.
- Cho CW, Kim DB, Shin SC. Development of bio adhesive transdermal bupivacaine gels for enhanced local anaesthetic action. IranJ Pharma Res 2012;11:423-431.
- Hoizey G., Lamiable D., Robinet A., et al. Sensitive bioassay of bupivacaine in human plasma by liquid-chromatography-ion trap mass spectrometry. J Pharma Biomed Anal 2005;39:587-592.
- Houri-haddad Y., Soskolne WA., Halabi A., et al. Repeat bacterial challenge in a subcutaneous chamber model results in augmented tumour necrosis factor-α and interferon-γ response, and suppression of interleukin-10. Immunol 2000;99:215-220.
- Mizrahi B., Shapira L., DombAJ., et al. Citrus oil and MgCl2 as antibacterial and anti-inflammatory agents. J Periodontol 2006;77:963-968.
- Sidhu P., ShojaeeAliabadi F., Andrews M., et al. Tissue chamber model of acute inflammation in farm animal species. Res Vet Sci 2003;74:67-77.
- Sokolsky-Papkov M., Golovanevski L., DombAJ., et al. Poly(DL:lactic acid-castor oil) 3:7-bupivacaine formulation: reducing burst effect prolongs efficacy in vivo. J Pharm Sci 2009;99:2732-2738.
- Golovanevski L., Ickowicz D., Sokolsky-Papkov M., In vivo study of an extended release bupivacaine formulation following site-directed nerve injection. J BioactivCompat Polymer: BiomedAppl 2014.
- Tong Leung VK., Suen SS., Singh Sahota D., et al. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J MaternFetal Neonatal Med. 2012;25:1774-1778.
- Sevilla R., Quintela O., De Diego C., et al. Development of an experimental porcine model of bupivacaine intoxication: correlation of plasma levels of bupivacaine and electrocardiographic parameters: 8AP3â€ÂÂ10. Europe J Anaesthesiol 2013;30:124-125.
- Shi K., Xia Y., Wang Q., et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. AnesthAnalg. 2013;116:804-809.
- Dal Bo L., Mazzucchelli P., Marzo A., Highly sensitive bioassay of lidocaine in human plasma by high-performance liquid chromatography-tandem mass spectrometry. J Chromatograph 1999;854:3-11.
- KauYC., Liao CC., Chen YC., et al. Sustained release of lidocaine from solvent-free biodegradable poly[(d,l)-lactide-co-glycoside] (PLGA): In vitro and in vivostudy. Material 2014;7:6660.
- Fettiplace M., Ripper R., Lis K., et al. Abstract 191: During lipid resuscitation, lipid-blood-fraction rapidly transports radiolabelled-bupivacaine from highly perfused organs to liver for accelerated processing and excretion. Circulation2013;128:191-198.
- Curley J., Castillo J., Hotz J., et al. Prolonged regional nerve blockade. Injectablebiodegradable bupivacaine/polyester microspheres. Anaesthesiol 1996;84:1401-1410.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences