ISSN : 2321-2748
American Journal of Phytomedicine and Clinical Therapeutics
Development and Validation of an HPLC-DAD Method for the Determination of Coumarin in Syrups with Guaco and Critical Analysis of Drug Labels
Carolina Passarelli Gonçalves1, Flavio Sussumu Yasuda1, Maria Aparecida dos Santos1, Romulo Dragani Reis1, Maria Isabel Savino1, William Ribeiro1, Luciane Reche1, Ivair Donizete Gonçalves1, Luis Carlos Marques2 and Maria Cristina Marcucci3*
1 Post-Graduate School in Pharmacy and Biotechnology, Anhanguera University of Sao Paulo, Brazil
2 Fitoscience Consulting Limited, Sao Paulo, Brazil
3 Laboratory of Natural Products and Chemometry, Anhanguera University of Sao Paulo, Brazil
- *Corresponding Author:
- Maria Cristina Marcucci
Post-Graduate, School in Pharmacy and Biotechnology
Anhanguera University of Sao Paulo, Sao Paulo, Brazil.
Tel: 005511-3512-8400
E-mail: cristina.marcucci@anhanguera.com
Received Date: October 11, 2017; Accepted Date: October 16, 2017; Published Date: October 20, 2017
Citation: Gonçalves CP, Yasuda FS, dos Santos MA, Reis RD, Savino MI, et al. (2017) Development and Validation of an HPLCDAD Method for the Determination of Coumarin in Syrups with Guaco and Critical Analysis of Drug Labels. Am J Phytomed Clin Ther Vol. 5 No. 3:19. DOI: 10.21767/2321-2748.100332
Abstract
We carried out an investigation with the purpose to evaluate the real concentration of coumarin by high performance liquid chromatography with diode array detector (HPLCDAD) in some guaco syrups and associations (Mikania glomerata Spreng.), marketed in Sao Paulo, Brazil. At first, we developed some analytical method parameters and then we investigated the quality of nine commercial syrups, purchased in pharmacies, compounding pharmacy and another in a house of Northeast herbals, called “lambedor of guaco, composed of natural herbs”. We also carried out an investigation about package and drug label of the studied products, according to ANVISA standardization. The most syrups samples have a lower coumarin concentration than those recommended by ANVISA as a minimum daily dose. Regarding to packaging and drug label, many of them are complies with the current legislation, while others are in a complete disagreement. Despite the importance of this plant species for the Brazilian herbal medicine, including those belonging to the official list of the Unified Health System, many efforts are still needed to offer to the Brazilian population adequate quality products. Therefore, it is necessary a better supervision about quality control of phytomedicines.
Keywords
Natural product; HPLC-DAD; Coumarin; Mikania glomerata
Introduction
The use of medicinal plants and herbal medicines has increased considerably. We have looking for searching of medicinal plants and their derivatives as natural therapeutic agents. To achieve those targets, herbal production necessarily requires prior studies on the botanical aspects, agronomic, phytochemicals, pharmacological, toxicological, development of analytical and technological methodologies [1]. The Brazilian National Health Surveillance Agency (ANVISA) has, over time, published guidelines to help the herbal industry, wherein currently some standards took effect recently. The current legislation aims to regulate and formalize the development and the use of herbal medicines in order to oppose the expression is commonly hear as users as some health professionals who, at the time of prescription and dispensing, refers “... what is natural has no side effects ...”. This statement is misleading and refers to numerous risks to public health. It was observed that numerous plants which phytochemical and pharmacological studies are potentially satisfactory, however the species in question does not even has agronomic and ecological research concerned with the reproduction of the plant or even sustainable extraction. There are many researches with numerous Brazilian plant species, which are objects of study in graduate programs across the country [2- 5]. On the other hand, the national herbal industry has numerous drugs marketed for decades based only in popular use. Efforts have been made to find and isolate phytochemical markers and realize pharmacological, pre-clinical and clinical studies, as in the case of guaco (Mikania glomerata), which traditional use can be considered almost secular [6]. The herbal production assumes that development studies have been carried out previously and the procedures and processing steps must to be well established. Fulfilling this regard, obtaining herbal products requires specific knowledge and skills in the medicinal products. Such knowledge and skills should be related each other, aiming the suitable of pharmaceutical products producing, according to current quality concepts. Therefore, the knowledge of what is intended to be combined with the standards, which would achieve the objective stroke, to achieve total quality [7]. Originally from South America, guaco predominates in Brazil south and southeast regions, in the forests and savannas. Guaco belongs to the Compositae family and has its distribution as native species in Brazil southern, from Sao Paulo to Rio Grande do Sul. It is a fickle, glabra climbing. Leaves simple, opposite, ovate and oblong-lanceolate, obtuse base and acute apex, up to 15 cm long and 7 cm wide, with three well evident ribs, petiolate, fleshy-leathery, bright green on the upper side, more pale at the bottom. Hermaphrodites flowers, gathered at number four chapters, equal to each other, white and papus tubulosa corolla, white-cream color; chapters grouped in branches espiciforms congested, or glomeruli (Figure 1A). The fruit is achene, glabrous. This plant is also known as grass-of-snakes, vine-scrub grass-of-charge, heart-of-jesus and guaco-smooth, Leaves of guaco are widely used by Brazilian indians in the form of poultice, they are macerated and applied about snake bites and other venomous animals [8,9]. As chemical composition, guaco has sesquiterpene and diterpenic compounds, stigmasterol, flavonoids, coumarins, resins, tannins, saponins, guacosides and chlorogenic acid [6,10-12]. Guaco (Mikania glomerata Spreng.) is a medicinal plant widely used for cough and respiratory diseases like flu, hoarseness, throat infection, bronchitis and tuberculosis [13-18]. Figure 1B shows the dried vegetable drugs. Studies reported the anti-edema activities and acute toxicity of the extract of Mikania glomerata ( M. glomerata) and M. laevigata in edema induced by carragenin in male Wistar rats paw. In the end of the study, the conclusion was that both extracts have inhibitory activity in the induced edema, but caution was needed to say that it is only because of guaco, since ethanol contained in the extract may exert a similar effect to the components of the plant. Pharmacological activities such as hypoglycemic, antidiarrheal, anti-Trypanosoma cruzi, antioxidant, allelopathic, larvicidal, anticonvulsant and antimalarial are also described in the literature for the Mikania genus plants [19-26]. Guaco also has expectorant and bronchodilators properties and it is indicated in respiratory conditions such as bronchitis, respiratory infections, colds and flu. Moreover, it is still commonly used to treat hoarseness, rheumatism, intestinal infections and to heal wounds [14,15,27,28]. The guaco chemical marker is the coumarin [29,30] (Figure 1C). Some analytical methods were developed and validated for the quantification of coumarin in guaco by spectrophotometry and HPLC-UV [31]) and HPLCUV with confirmation of the presence of coumarin by mass spectrometry [32]). The aim of this work was to develop and validate an HPLC-DAD method with the purpose to evaluate the real concentration of coumarin in some guaco syrups and associations. We also carried out an investigation about package and drug label according of Brazilian agency of health.
Materials and Methods
Materials and reagents
The coumarin standard (1,2-benzopyrone) was purchased from Sigma-Aldrich (USA). All reagents used were HPLC grade. The water used as mobile phase and dissolution of standard and syrups was ultrapure (Barnsted system VWR, USA, 18.3 Ohms). The guaco syrups (nine) were purchased in a local trade. Eight commercial syrups were obtained in pharmacies and other in a Brazilian Northeastern House, called "lambedor of guaco, composed of natural herbs". Syrups 3 and 5 are associations of guaco with watercress, Polygala (Polygala senega), Tolu balsam (Myroxylon balsamum or M. balsamum var. pereirae), Ipeca (Cephaelis ipecacuanha) and Aconite (Aconitum napellus). The “lambedor” has natural scent of honey, copaiba oil (Copaifera langsdorffii) and guaco extract.
Plant material: Adult specimens of M. glomerata were obtained from CPQBA UNICAMP (Campinas State University, Campinas, SP, Brazil) identified by Dr. Caetano Troncoso de Oliveira and vouchers deposited in the UNICAMP Herbarium under numbers UEC 102046 and 102047. The specimens are part of a joint project with Dr. ACF Sawaya, from UNICAMP [33]).
Instrumentation: We use an analytical balance (Ohaus Co, USA 0.00001 g/0.0001) and a high performance liquid chromatography equipment (LaChrom, model D-7000 from Merck, Darmstadt, Germany) connected to a diode array detector (LaChrom, model D-7100) (HPLC-DAD) and the program used for data analysis was the Merck-Hitachi model D7000 (Chromatography data Station - DAD Manager-Merck, Darmstadt, Germany).
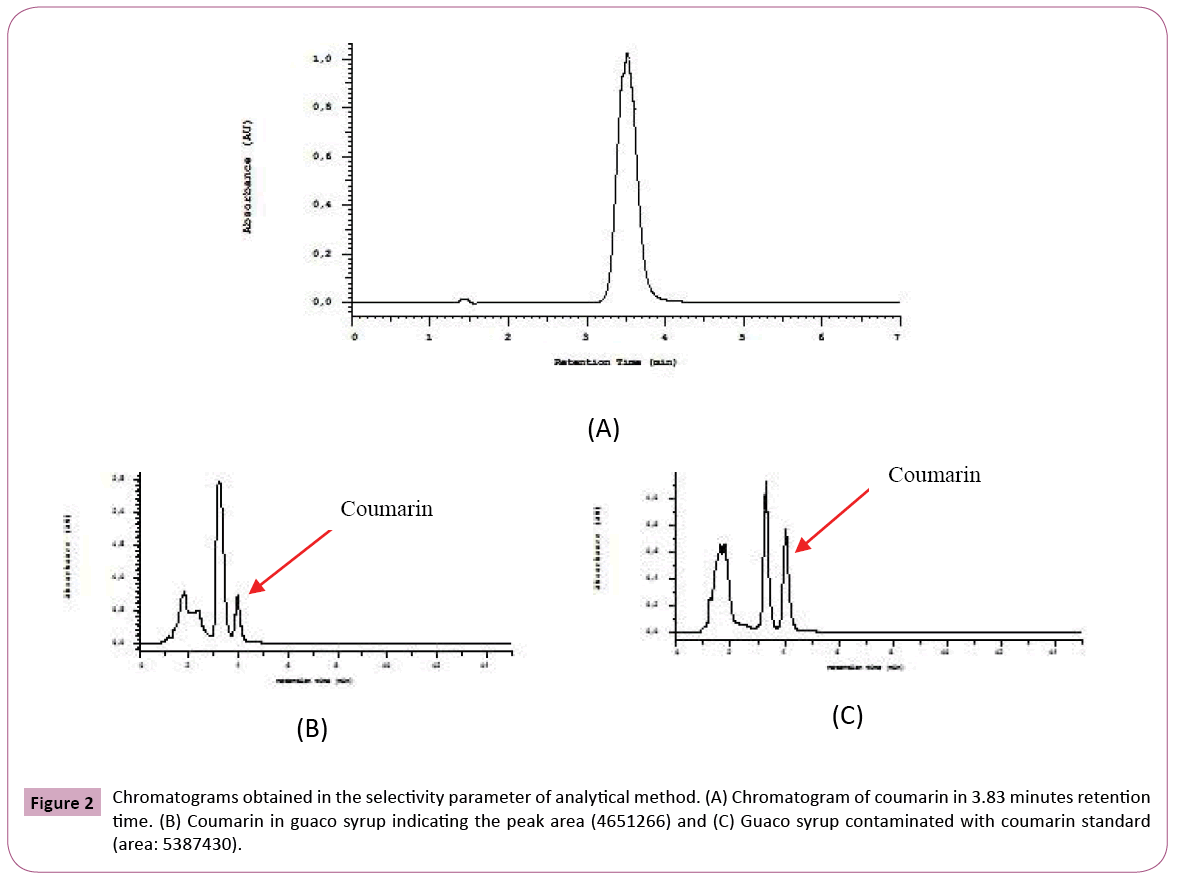
Chromatographic conditions: The coumarin standard and guaco syrup (ten samples) were analyzed by HPLCDAD according to the following method: isocratic method (50% of water with 5% of formic acid (A) and 50% methanol (B)), C18 reverse phase column Lichrochart (5 μm) 125 mm (Merck, Darsmtadt, Germany) flow 0.8 mL/min. Detection was at 275 nm and the run time of 7 minutes. The coumarin retention time was 3.8 minutes.
Analytical validation: We evaluated parameters in the validation of the analytical method like selectivity, precision, accuracy, range, linearity, residuals, detection and quantification limits, according to the standards proposed in Resolution No. 899 [34,35].
Selectivity: The selectivity was evaluated by comparing the injection of the blank (mobile phase) with coumarin (40 mg/mL), guaco syrup and guaco syrup contaminated with coumarin. The analysis was performed in triplicate.
Precision: The repeatability of the analytical method was evaluated by injecting six independent preparations of a guaco syrup, on the same day by the same analyst. Obtaining the results, we calculated the mean, standard deviation (SD) the coefficient of variation (CV%) and simple uncertainty (Inc.). The analysis was performed in sextuplicate.
Accuracy: Accuracy was evaluated by contamination (spike) of the sample of "lambedor of guaco" in only one level of coumarin concentrations, 50 μg/mL. The analysis was performed in triplicate.
Linearity: A calibration curve was prepared with volumetric flasks of 10 mL calibrated at the concentrations (μg/mL): 10.0; 20.0; 30.0; 40.0; 50.0; 60.0; 70.0 and 80.0, from standard stock solution with a concentration 933 mg/mL of coumarin in water ultrapure.
Quantification of coumarin in syrups: About 3 mL of guaco syrup was used to quantify the coumarin content. This quantity was transferred for a 10 mL beaker, filtered through filter paper and glass funnel into another 10 mL beaker. Filtered to vial using a syringe and a 0.45 μm filter (Sartorius, USA). The calculation of the coumarin content in the syrup was made in Validation Manager software (Merck, Darmstadt, Germany). Three vials from each sample were prepared and injected in triplicate. The syrup densities were measured by pycnometer at 25°C.
Statistical analysis: For the analysis of the differences between the samples, we used the Analysis of Variance (ANOVA) followed by Tukey - Kramer test for multiple comparisons using the Prism 5.0 software (GraphPad Software Inc CA USA).
Packaging and drug label analysis: The packages were analyzed by a questionnaire conducted from the standards of ANVISA [36]. According to the drug label standards, the legislation advocates the drug label is the main instrument that allows the patient to know exactly how to use and how to avoid the risks of drug consumption prescribed by the health professionals [37].
Results and Discussion
Analytical validation parameters
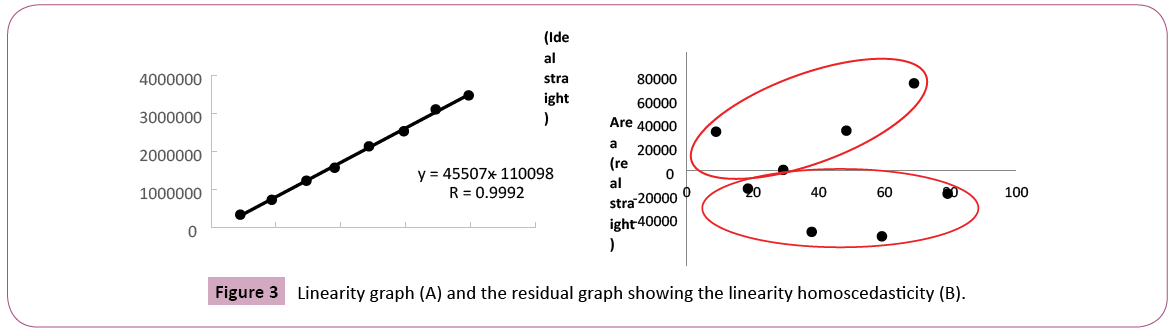
The concentration of coumarin in commercial guaco syrups was evaluated through analytical method who is presented in this work. Some analytical validation parameters were evaluated. The selectivity is the ability of a method to quantify the analyte accurately in the presence of interferences existing in the sample, such as impurities, degradation products and excipients. With respect to an herbal, selectivity is a very important parameter because it can show that the marker or markers can be detected unequivocally. As those herbals are complex matrices and you cannot compare with placebo, this parameter is indispensable [38]. Figure 2 shows chromatograms of standard and sample contaminated with standard to the selectivity evaluation. The solvent chromatogram did not show the appearance of a peak. In this study, the analytical method developed for the quantification of coumarin, was selective because no peak appears in the default retention time, taking into account the race time of the mobile phase, the standard and the sample contaminated with standard. Several analytical methods have been proposed for determination and quantification of coumarin from different sources. Soares and Silva [31] proposed an analytical method where the separation was done in the isocratic mode, using acetonitrile: water (40:60; v/v) at a flow rate of 1 mL / min. Lachenmeier [39] described a method using mobile phase A (water, 5 mM ammonium acetate bufter, 0.2% (v/v) acetic acid) and mobile phase B (acetonitrile / methanol 1: 2 (v/v)) in a gradient program with a 0.2 ml / min: 0-2 min: 70% A; 2-20 min: 70-20% A; 20-22 min: 20-70% A; 22-30 min: 70% A. In the method developed by Scotter et al. The gradient elution was performed using mobile phase A (acetonitrile) and mobile phase B (0.5% acetic acid, v/v). The gradient program was 25: 75 (A: B) for 9 min then to 80: 20 (A: B) over 11 min. This was followed by a reverse gradient over 0.2 min held at 25: 75 (A: B) for 8 min. Then Jin [40] proposed a method without the use of acetonitrile. The mobile phase consisted of methanol (A) and 0.05% acetic acid aqueous (B) with gradient elution as follows: 0-3 min, 40-40% A; 3-8 min, 40-65% A; 8-10 min, 65-95% A; 10- 12 min, 9595% A and then returned to the initial condition. The flow rate was 0.8 mL / min, and the injection volume was 20 μL for analysis. The reagent used in the HPLC method, acetonitrile, is teratogenic with a toxic profile by several routes of absorption and the gradient method that can increase the cost of the analysis, but in the elution gradient, the price and complexity of the analysis can increase. The disadvantage of most of the methods already described is the use of acetonitrile, which is teratogenic with a toxic profile by several routes of absorption and the use of elution gradient, which makes the analysis more expensive and complex. This new method developed is novel, economical, rapid and specific for the assay of that active ingredient coumarin. The developed method has been validated and showed excellent linearity, accuracy, precision, selectivity and system suitability. With respect to the accuracy, a characteristic that expresses the degree of dispersion or similarity obtained when a homogeneous sample is scanned repeatedly, using the same method [41] it gave us acceptable values, according to the US Pharmacopoeia with a standard deviation of relative value (SDR) recommended no more than 2%. In the precision parameter for the standard concentration was found a value of SDR of 0.001% and 1.55% for peak area (Table 1). Accuracy is how close that exists between the amount accepted as right and the value determined in the method. In this case, recovery assay can be performed. The recovery (or restoration factor) R, is defined as the ratio of the amount of the substance of interest present or added in the analytic portion of the test material which is capable of being extracted and quantified [38]. We used only one level of accuracy, one sample of "Lambedor of guaco" were contaminated with of 50 μg/mL of coumarin. The result obtained for the accuracy, was 100.5 ± 0.72%, simple uncertainty of 0.41%. It indicates, according to the Brazilian standards and international that the method is accurate. In this case, we used only one level of contamination to be sure that the lack of coumarin in the product is not due to an error of methodology, but the products does not have a guaco extract in the formulation. The mean and standard deviation was calculated, we obtained an average analytical curve. The straight-line equation was obtained by the method of least squares, y=45507x-110098, in a range from 10.0 to 80.0 μg/mL (Figure 3A). By linearity, obtained through calibration curve according to ANVISA criteria that the minimum acceptable value of the correlation coefficient is 0.98 for an herbal (Brazil, 2003), we obtained a value of 0.9920. It was demonstrated that a correlation exists between area and concentration, as evidenced in the residual plot (Figure 3B) where we can prove homocedastity and the residues normally distributed [42]. We evaluated the sensitivity of the chromatographic method by determining the limits of detection (LOD) and quantification (LOQ) and the values obtained were 0.90 μg/mL and 2.72 μg/mL, respectively.
| Concentration(µg/mL) | Area | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Average CV% | SD | CV% | Inc | Area | Average | SD | CV% | Inc |
| 79.37 | 3547448 | ||||||||
| 79.46 | 3455699 | ||||||||
| 79.46 | 79.43 | 0.05 | 0.001 | 0.001 | 3452488 | 3485212 | 53922 | 1.55 | 31132 |
SD is the standard deviation, %CV 3 is the coefficient of variation (%) and Inc. simple uncertainty calculated as the ratio between the standard deviation and the square root of the number of measures (3).
Table 1: Analytical method accuracy parameters.
Quantification of coumarin in syrups
Table 2 shows the amount in mg/mL of coumarin in the analyzed syrups. Quantification of coumarin in syrups and “lambedor of guaco” showed a quite varied concentrations, and the sample 6 showed a significant difference (P<0.0001) in comparison to other samples of syrup due to it has the major coumarin content. Samples 4, 6, 7, and 8 are significantly different from samples 1, 2 and 3 (P<0.0001). The sample 5 and “lambedor of guaco” are significantly different from the others (P<0.05) due to lack of coumarin marker in this samples.
| Syrup | mg/mL# | µg/mL* | SD | CV% | Inc. |
|---|---|---|---|---|---|
| 1 | 0.0070 | 6.96 | 0.04 | 0.57 | 0.02 |
| 2 | 0.0051 | 5.09 | 0.09 | 1.77 | 0.05 |
| 3(+Association) | 0.0034 | 3.35 | 0.04 | 1.12 | 0.02 |
| 4 | 0.0564A | 56.44 | 0.07 | 0.13 | 0.04 |
| 5(+Association) | 0.0000B | 0.00 | 0.00 | 0.00 | 0.00 |
| 6 | 0.1278A | 121.29 | 6.08 | 4.76 | 3.51 |
| 7 | 0.0332A | 32.54 | 0.61 | 1.83 | 0.35 |
| 8 | 0.0298A | 29.80 | 0.16 | 0.54 | 0.09 |
| “Lambedor” (+Association) | 0.0000B | 0.00 | 0.00 | 0.00 | 0.00 |
| “Lambedor”+coumarin§ | 0.0493 | 49.29 | 0.72 | 1.43 | 0.41 |
#Value for daily dose calculation, as recommended by ANVISA (Brazil). *We considered as the precision of the analytical method, the injection of each syrup sample, three preparations and injected three times each one. Abbreviation, see Table 1. We considered contaminated sample to the accuracy assay. The samples 4, 6, 7 and 8 are different from samples 1, 2 and 3 (P<0.0001). BThe sample 5 and “Lambedor” are significantly different from the others (P<0.05).
Table 2: Quantification of coumarin (in μg/mL) in syrups and fortification of “Lambedor”.
To comply with the daily intake range of coumarin treatment as advocated by ANVISA (0.5-5.0 mg) [43,44]), in some syrups, it is necessary to intake more than one liter of syrup per day (Table 3) although it has been reported the safety its use in certain doses [16]. With respect to packaging, all syrups, except the "Lambedor" are complies with the ANVISA´s specification, as described in Tables 4 and 5. The "Lambedor" has on the bottle some parameters of "nutritional value" that are no complies with the standards for herbal medicines [44,45]. The company mentions on the packaging that the product, which is an association (natural aroma of honey, copaiba oil, guaco extract and sucrose), was registered as a food supplement [46,47] Produced by technicians professional in laboratories, the drug labels do not always follow consistent standard and reading facilitators for the patient, for example, excessive use of technical terms and the complex and confusing syntax. However, it is an important guiding tool to patients for the correct use of medicines, including herbal medicines. There are some of the problems that occur in the Brazilian market of herbal products, which need to be solved in order to permit the adequate increased of phytotherapy.
| Syrup | Dose (mL) | Minimum dose* | Maximum dose* |
|---|---|---|---|
| 2 | 98 | 982 | |
| 3 | 149 | 1491 | |
| 4 | 9 | 89 | |
| 5 | ? | ? | |
| 6 | 4 | 39 | |
| 7 | 15 | 151 | |
| 8 | 17 | 168 | |
| lambedor? | lambedor? | ? |
Table 3: Syrup daily dose (mL) to attend the actual legislation.
| Description for primary packing analysis* | YES%(a) | NO%(b) |
|---|---|---|
| Generic name of the active substance in lowercase or botanical nomenclature (genus and species) in the case of herbal medicines | 90 | 10 |
| Active ingredient concentration per dosage unit, with the exception of multivitamins and/or poliminerals and/or amino acids | 90 | 10 |
| Record holder's name or logo as long as it contains the company name | 90 | 10 |
| Batch number and expire date (month/year) | 90 | 10 |
| Route of administration | 90 | 10 |
| Costume servisse | 100 | 0 |
| Drug label content** | YES%(a) | NO%(b) |
| Use CAPS and bold to highlight the questions and the bull of items; | 90 | 10 |
| It must have underlined and italicized text only for scientific names | 90 | 10 |
| It must to be printed with black letters on a white paper that does not allow the print visualization of the bull´s other side, when it is on a surface. | 90 | 10 |
| Medicine identification | YES%(a) | NO%(b) |
| Mention the medication commercial or trade name | 90 | 10 |
| For herbal medicines, inform plant species and plant part used | 65 | 35 |
| Presentation | ||
| Farmaceutical form | 90 | 10 |
| The concentration per unit or pharmacotechnique unit, as appropriate; | 90 | 10 |
| The total amount of weight, liquid volume or pharmacotechnique unit as appropriate; | 90 | 10 |
| Include the phrase, for CAPS and bold, "ADULT USE UP _____" and / or "PEDIATRIC USE UP ____", indicating the minimum age in months or years, for which indication was approved on record. | 65 | 35 |
| Composition | YES%(a) | NO%(b) |
| For herbal medicines, inform the composition of the product, indicating the real relationship in weight or volume, the vegetable raw material used the equivalent markers and the derivate description. | 90 | 10 |
| Information to patients | ||
| What does this medicine indicated for? | 65 | 35 |
| How does this medicine act? | 65 | 35 |
| When must I not use this medicine? | 65 | 35 |
| What must I know before using this medicine? | 65 | 35 |
| Where, how and how long can I storage this medicine? | 65 | 35 |
| How must I use this medicine? | 65 | 35 |
| What must I do if I forget to take this medicine? | 65 | 35 |
| What are the evils that this medicine can cause me? | 65 | 35 |
| What must I do if someone takes a larger quantity than indicated for this medicine? | 65 | 35 |
Table 4: Legal criteria for packaging and drug labels presentation: product numbers in complies (Yes) or not complies (No). (Yes) or not complies (No).
| Technical information for health professionals | YES%(a) | NO%(b) |
|---|---|---|
| Indication | 90 | 10 |
| Efficacy results | 65 | 35 |
| Pharmacological characteristics | 65 | 35 |
| Contraindications | 65 | 35 |
| Warnings | 65 | 35 |
| Interactions | 65 | 35 |
| Storage warnings | 65 | 35 |
| Dose and methods of use | 90 | 10 |
| Side effects | 65 | 35 |
| Overdose | 65 | 35 |
| Legal Information | YES%(a) | NO%(b) |
| Report the letters "MS", the registration number in the Ministry of Health as published in the Union Official Diary (DOU), requiring the nine (9) leading digits. | 90 | 10 |
| Inform the name, registration number and initials of the responsible technical holder of the record company Regional Pharmacy Council. | 90 | 10 |
| Inform the corporate name and the registrant business address in Brazil. | 100 | 0 |
| Report the number of the TAX ID (CNPJ) of the registrant. | 100 | 0 |
| Inform the telephone Customer service (SAC), the holder of the record company's responsibility. | 0 |
Table 5: Legal criteria for packaging and drug labels presentation: technical information for health professionals (product numbers in complies (Yes) or not complies (No)).
Conclusion
An HPLC method was developed, which has been validated and shown applicable to the determination and quantification of coumarin in guaco syrups. The method was validated according to ANVISA and ICH standards for this purpose, showing selectivity, precision, linearity and accuracy. Limits of detection and quantification were determined. With reference to the quantification of coumarin in syrups, it has been shown that some of them have amount below parameters established by ANVISA as a minimum dose of daily intake, requiring exacerbated quantities of product to produce the desired therapeutic effects. With respected to the drug labels and packaging, most products met the requirements of the legislation, but the "lambedor" did not attend the majority of legal requirements required by the regulatory agency as constant packaging and drug labels of herbal medicines. Thus, despite the importance of this plant species for the Brazilian herbal medicine, including those mentioned in the official list of the Unified Health System, many efforts are still needed to offer to the Brazilian population adequate quality products. The success of phytotherapy depends, among other factors, of maintaining a minimum quality control of the products, no production of therapeutic effects is due to absence of active ingredients, testifying against the own herbal market.
References
- Soejardo DD (1996) Biodiversity prospecting and sharing: from the field. JEthanopharmacol 51: 1-15.
- Amaral FMM, Coutinho DF, Ribeiro MNS, Oliveira MA(2003)Evaluation of the quality of vegetal drugs marketed in São Luis - Maranhão Rev Bras Farmacogn 13: 27-30.
- Melo JG, Nascimento VT, Amorim ELC, Lima ACS, Albuquerque UP, et al. (2004) Quality evaluation of commercial samples of boldo, cow's foot and ginco. Rev.Bras.Farmacogn 14: 111-120.
- Mariath IR, Falcao HS, Barbosa-Filho JM(2009)Plants of the American continent with antimalarial activity. Rev Bras Farmacogn19: 158-192.
- Tobias ML, Oliveira F, Oliveira KP, Marques LC (2007) Quality control of plant drugs from pharmacies in Maringá (Paraná, Brazil). Rev Eletr Farm 4: 95-103.
- Czelusniak KE, Brocco A, Pereira DF, Freitas GBL (2012) Farmacobotânica, fitoquímica e farmacologia do guaco: revisão considerando Mikania glomerata Sprengel e Mikania laevigata Schulyz Bip. ex Baker. Rev Bras Pl Med14: 400-409.
- Carvalho ACB, Nunes DSG, Baratelli TG(2007)Aspects of legislation in the control of herbal medicines. T&C Amazon5: 26-32.
- Brandao MGL, Cosenza GP, Moreira RA (2006) Medicinal plants and other botanical products from the Brazilian Official Pharmacopoeia. Rev BrasFarmacogn16:408-420.
- Brandao MGL, Zanetti NNS, Oliveira GRR (2008) Other medicinal plants and botanical products from the first edition of the Brazilian Official Pharmacopoeia. Rev Bras Farmacogn18: 127-134.
- Gasparetto JC, de Francisco TMG, Pontarolo R (2013) Chemical constituents of Mikania glomerata Spreng and Mikania laevigata Sch Bip ex Baker. J Med Plants Res 7: 753-765.
- Passari LMZG, Scarminio IS, Bruns RE(2014) Experimental designs characterizing seasonal variations and solvent effects on the quantities of coumarin and related metabolites from Mikania laevigata. Anal Chim Acta 821: 89-96.
- De Melo LV, Sawaya ACHF (2015) UHPLC–MS quantification of coumarin and chlorogenic acid in extracts of the medicinal plants known as guaco (Mikania glomerata and Mikania laevigata). Rev Bras Farmacogn25: 105-110.
- Di Stasi LC, Oliveira GP, Carvalhaes MA(2002)Medicinal plants popularly used in the Brazilian Tropical Atlantic Forest.Fitoterapia 73: 69-91.
- Silva MIG, Gondim APS, Nunes IFS, Sousa FCF(2006)Utilization of phytotherapics in the basic health care units of the family in the municipality of Maracanaú (CE). Rev Bras Farmacogn 16: 455-462.
- Tavares JP, Martins IL, Vieira AS, Lima FAV (2006) Clinical toxicology study of a herbal medicine based on associations of plants, honey and propolis. Rev Bras Farmacogn16: 350-356
- Graça C, Freitas CS, Baggio CH, Dalsenter PR (2007) Mikania laevigata syrup does not induce side effects on reproductive system of male Wistar rats. JEthnopharmacol111: 29-32.
- Graça C, Baggio CH, Freitas CS(2007)In vivo assessment of safety and mechanisms underlying in vitro relaxation induced by Mikania laevigata Schultz Bip. ex Baker in the rat trachea. JEthnopharmacol112:430-439.
- Leitao F, Leitao SG, De Almeida MZ(2013)Medicinal plants from open-air markets in the State of Rio de Janeiro, Brazil as a potential source of new antimycobacterial agents. J Ethnopharmacol 149: 513-521.
- BarbosaJM, Vasconcelos THC, Alencar AA(2005)Plants and their active constituents from South, Central, and North America with hypoglycemic activity. RevBrasFarmacogn15: 392-413.
- Salgado HRN, Roncari AFF, Moreira RRD (2005) Antidiarrhoeal effects of Mikania glomerata Spreng. (Asteraceae) leaf extract in mice. Rev Bras Farmacogn. 15: 205-208.
- Saúde DA,Faria AR (2007)Substances of the nature with anti-Trypanosoma cruzi activity. Rev Bras Farmacogn17:455-465.
- Vicentino ARR, Menezes FS (2007)Antioxidant activity of vegetable dyes, sold in pharmacies with manipulation and indicated for several types of diseases by the DPPH methodology. Rev Bras Farmacogn 17: 384-387.
- Baratto L, Lang KL, Vanz DC (2008) Investigation of the allelopathic and antimicrobial activities of Mikania laevigata (Asteraceae) obtained from hydroponic and traditional cultures. Rev Bras Farmacogn 18: 577-582.
- Quintans LJ, Almeida JRGS, Lima JT (2008) Plants with anticonvulsant properties - A review. Rev Bras Farmacogn18: 798-819.
- Mariath IR, Falcao HS(2009)Plants of the American continent with antimalarial activity. Rev Bras Farmacogn19: 158-192.
- Araújo AL, Lacerda T, Hiura E(2016)Larvicidal potential of Mikania glomerata SPRENGEL extract on Ancylostoma caninum larvae. Afr J Pharm Pharmacol 10: 379-384.
- Osório AC, Martins JLS(2004)Determination of coumarin in fluid extract and guaco dye by spectrophotometry derived from first order. Rev Bras Cienc Farm40: 481-486.
- Da Silva CR, Gomes VS, Kulkamp IC (2008) Spectroscopic method for determination of coumarin in Mikania glomerata Sprengel syrup. Rev Bras Farmacogn 18: 594-599.
- Bolina RC, Garcia EF, Duarte MGR(2009)Comparative study of the chemical composition of plant species Mikania glomerata Sprengel and Mikania laevigata Schultz Bip. Ex Baker. Rev Bras Farmacogn.19: 294-298.
- DeMelo LV, Sawaya ACHF (2015) UHPLC–MS quantification of coumarin and chlorogenic acid in extracts of the medicinal plants known as guaco (Mikania glomerata and Mikania laevigata). Rev Bras Farmacogn25: 105-110.
- Soares SL, Da SLS, Brumano L(2012)Preparation of dry extract of Mikania glomerata Sprengel (Guaco) and determination of its coumarin levels by spectrophotometry and HPLC-UV. Molecules 17:10344-10354.
- Bertoldi FC, Deschamps FC (2016)Validation of a fastanalytical method by CLAE-UV for determination of coumarin in guaco (Mikania glomerata Sprengel) confirmed with mass spectrometry. Rev Bras Pl Med 18: 316-325.
- Costa VCO, Borghi AA, Mayer JLS (2017)Comparison of the morphology, anatomy, and chemical profile of Mikania glomerata and Mikania laevigata. Planta Med19:1-10.
- Resolução nº 899(2003) de 29 de maio. Guia para validação de métodos analíticos e bioanalíticos. Agência Nacional de Vigilância Sanitária, Ministério da Saúde. Poder executivo, Brasília, DF, Diário Oficial da União, 02 Jun 2003, Brazil.
- International Conference on Harmonization (ICH) (2005) Validation of Analytical procedure: Text and Methodology Q2 (R1). Geneva: IFPMA, 2005.
- Agencia Nacional de Vigilância Sanitária (2013) Consolidado de normas da COFID. Agência Nacional de Vigilância Sanitária, Ministério da Saúde,Brazil, 2013.
- Resoluçao RDC 47(2009)de 08 de setembro. Regulamento de bulas aplicado a todos os medicamentos registrados ou notificados na Anvisa. Internet]. Agência Nacional de Vigilância Sanitária, Ministério da Saúde. Poder executivo, Brasília, DF, Diário Oficial da União, Brazil 2009.
- Marcucci MC (2011)Validation of analytical methods applied to medicinal and phytotherapeutic plants.
- Lachenmeier DW, Sproll C, Ruge W(2008)HPLC analysis and safety assessment of coumarin in foods. Food Chemistry 109:462-469.
- Jin S, Zhao M, Ding W(2016)Simultaneous determination of nine coumarins in rat plasma by HPLC–MS/MS for pharmacokinetics studies following oral administration of Fraxini Cortex extract. Journal of Chromatography B 1025: 25-32.
- Merck (2003)Validation of chromatographic methods. Germany.
- Ribeiro FAL, Ferreira MMC, Morano SC (2008) Validation worksheet: a new tool for estimating figures of merit in the validation of univariate analytical methods. Chem New 31: 164-171.
- Instrução Normativa (2014)nº 4, de 18 de junho. Guia de orientação para registro de Medicamento Fitoterápico e registro e notificação de Produto Tradicional Fitoterápico. Agência Nacional de Vigilância Sanitária. Ministério da Saúde. Poder executivo, Brasília, DF, Diário Oficial da, Brazil 2014.
- Instrução Normativa (2014) no. 2, de 13 de maio. Lista de medicamentos fitoterápicos de registro simplificado. Agência Nacional de Vigilância Sanitária. Ministério da Saúde. Poder executivo, Brasília, DF, Diário Oficial da União Brazil, 2014.
- Barnes J, Anderson LA, Phillpson JD(2002) Herbal medicines: a guide for healthcare professionals. 2nd edn. London: Pharmaceutical Press.
- Oliveira F, Saito ML, Garcia L (1993)Thin layer chromatographic characterization of guaco fluid extract Mikania glomerataSpreng. Lecta 11: 43-55.
- USP-The United States Pharmacopeia (2012) 35 ed. USP 35. Rockville: United States Pharmacopoeial Convention 35: 164.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences