ISSN : 2575-7725
Journal of Stem Cell Biology and Transplantation
Cure of Primary Plasma Cell Leukemia by Tandem Autologous HSCT Followed by Maintenance Therapy
Mokhtar N1, Al-Omari R1, AlHarbi S1, Mutahar E1, Raslan H2, Kalogiannidis P1 and Al-Anazi KA1*
1Department of Hematology and Hematopoietic Stem Cell Transplantation, King Fahad Specialist Hospital, Saudi Arabia
2Department of Pathology, Section of Hematopathology, King Fahad Specialist Hospital, Saudi Arabia
- *Corresponding Author:
- Khalid Ahmed Al-Anazi
Department of Hematology and Hematopoietic Stem Cell Transplantation, Oncology Center, King Fahad Specialist Hospital, Saudi Arabia
Tel: 966-03-8431111
Fax: 966-03-8427420
E-mail: kaa_alanazi@yahoo.com
Received date: March 29, 2019; Accepted date: April 18, 2019; Published date: April 26, 2019
Citation: Mokhtar N, Al-Omari R, AlHarbi S, Mutahar E, Raslan H, et al. (2019) Cure of Primary Plasma Cell Leukemia by Tandem Autologous HSCT Followed by Maintenance Therapy. J Stem Cell Biol Transplant Vol.3 No.1:2. doi: 10.21767/2575-7725.100023
Copyright: © 2019 Mokhtar N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Plasma cell leukemia is an aggressive hematologic malignancy with rapidly progressive course and poor clinical outcome. It requires aggressive induction therapy followed by allogeneic hematopoietic stem cell transplantation in patients who are fit and eligible for this form of therapy. We report a female patient who was diagnosed to have primary plasma cell leukemia in November 2015. Her disease was controlled by a combination of cytotoxic chemotherapy and novel agents. Due to absence of sibling donors for allogeneic transplantation, she received tandem autologous stem cell transplantation followed by 2 cycles of consolidation then continuous maintenance therapy. This line of management has already achieved a durable disease control and hopefully cures of her aggressive plasma cell malignancy.
Keywords
Plasma cell leukemia; Cytotoxic chemotherapy; Novel agents; Hematopoietic stem cell transplantation; Tandem transplantation; Maintenance therapy
Introduction
Plasma cell leukemia (PCL) is a rare and aggressive plasma cell neoplasm with rapidly progressive clinical course [1-10]. It is defined in the presence of >20% circulating plasma cells and/ or an absolute plasma cell count >2 × 109/L in a peripheral blood smear [2-9,11,12]. PCL can be: (1) primary or de novo which accounts for 60%-70% of all PCLs, or (2) secondary which represents leukemic transformation of multiple myeloma (MM) and accounts for 30%-40% of cases of PCL [1-3,7,9,11,12].
The clinical and laboratory manifestations of PCL include: occurrence at younger age; hypercalcemia; hyper leukocytosis; elevated beta2 microglobulin (B2M); elevated serum lactic dehydrogenase (LDH) level; low serum M protein level; high frequency of renal failure; extensive bone disease; extra medullary involvement such as hepatosplenomegaly and lymphadenopathy; increased incidence of bone marrow (BM) failure; distinct immunophenotypic features such as high expression of CD20, lack of CD 56 expression and low expression of CD 9, CD117 and HLA-DR; high-risk (HR) or adverse cytogenetic, high plasma cell labeling index; rapid disease progression; advanced international staging system stage at presentation; fast decline in performance status; and rapid relapse after treatment [3,4,9,10,13-15]. Unfortunately, both primary and secondary types of PCL have dismal outcomes with secondary PCL having worse prognosis than primary PCL [1-3,6,11].
Case Presentation
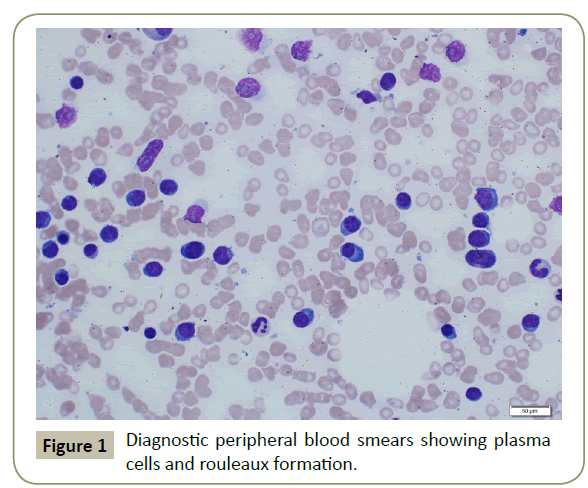
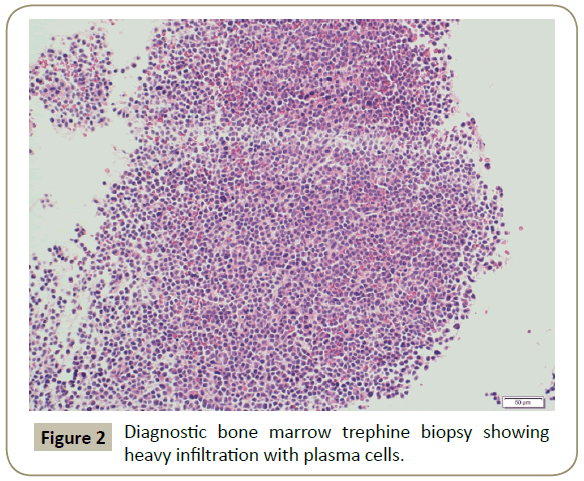
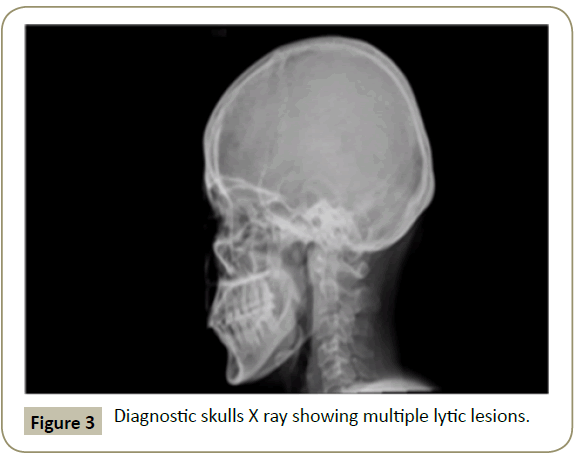
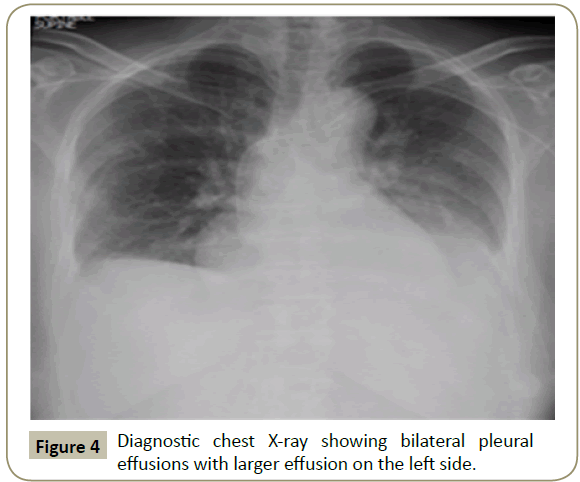
In November 2015, a 34 year old Saudi female presented to King Fahad Specialist Hospital (KFSH) in Dammam, Saudi Arabia with anorexia, malaise, weight loss, anemic symptoms, and bone pains for 4 months. She gave no history of any medical or surgical illness and she was not on any regular medication. Her physical examination at presentation revealed that the patient was in pain, having pallor and emaciation, but without jaundice, leg edema or external palpable lymphadenopathy. There was dullness on percussion and reduced air entry over the base of the left lung posteriorly. Her abdominal examination showed no tenderness or palpable organomegaly. Her cardiovascular and neurological examinations revealed no abnormality. Her CBC showed: white blood count (WBC): 74.32 × 109/L, hemoglobin: 7.1 g/dL, platelet count: 136 × 109/L. Differential cell count showed: 16% neutrophils, 13% lymphocytes and 1% monocytes in addition to 70% plasma cells. Peripheral blood film showed sheets of plasma cells and mild thrombocytopenia (Figure 1). Her serum creatinine was 157 μmol/L. Hepatic and coagulation profiles were normal. BM examination revealed: 90% cellularity and diffuse infiltration by plasma cells with strongly positive CD138, positive CD38 and lambda light chain restriction (Figure 2). Chromosomal analysis showed complex cytogenetic: (1) monosomies of chromosomes 8, 13 and 14; (2) translocation between the long arm of chromosome 3 at q25 and the distal short arm of chromosome 7 at p14; (3) translocation between the short arm of chromosome 1 at p13 and the distal long arm of chromosome 6 at q21; (3) additional materials of unknown origin on the distal long arms of chromosome 16 at q23 and chromosome 17 at q24. However, fluorescence in situ hybridization revealed negative p53 and ATM genetic mutations. Total protein: 137 g/L, serum calcium: 3.2 mmol/L, serum LDH: 2140 units/L, B2M: 11.1 mg/L and serum IgG: 92 g/L. Serum protein electrophoresis showed a peak in B2 fraction consistent with monoclonal band. Skeletal survey showed diffuse osteopenia and multiple lytic lesions involving the skull and long bones without pathological fractures (Figure 3) and chest X-ray showed bilateral pleural effusions with larger effusion on the left side (Figure 4). Analysis of the pleural fluid showed monoclonal plasma cells reflecting involvement by PCL.
Figure 1: Diagnostic peripheral blood smears showing plasma cells and rouleaux formation.
After establishing the diagnosis of primary PCL and controlling her renal dysfunction, anemia, hypercalcemia and bone pains, the patient was commenced on VTD-PACE regimen (a combination of bortezomib, dexamethasone, thalidomide, cisplatin, adriamycin, cyclophosphamide, and etoposide) in addition to monthly doses of zolendronic acid 4 mg intravenously (IV). After receiving 2 cycles of VTD-PACE regimen, she achieved complete remission (CR) of her PCL with no circulating plasma cells, ˂5% plasma cells in the BM, absence of extra medullary disease, and negative immunofixation on serum and urine samples.
As the patient had no siblings for allogeneic hematopoietic stem cell transplantation (HSCT), she was planned for tandem autologous HSCT instead. She received high-dose melphalan 200 mg/m2 IV on 25/01/2016 followed by the first autologous HSCT on 26/01/2016 using non-cryopreserved stem cells and the second autologous HSCT with melphalan conditioning 160 mg/ m2 IV on 01/04/2016 using cryopreserved stem cells. Thereafter, she received 2 cycles of consolidation treatment composed of bortezomib, lenalidomide and dexamethasone (VRD). In August 2016, the patient was commenced on maintenance therapy with bortezomib and dexamethasone. Cycles of maintenance therapy were administered every 2 weeks till September 2018. Thereafter, she was shifted to single agent lenalidomide maintenance.
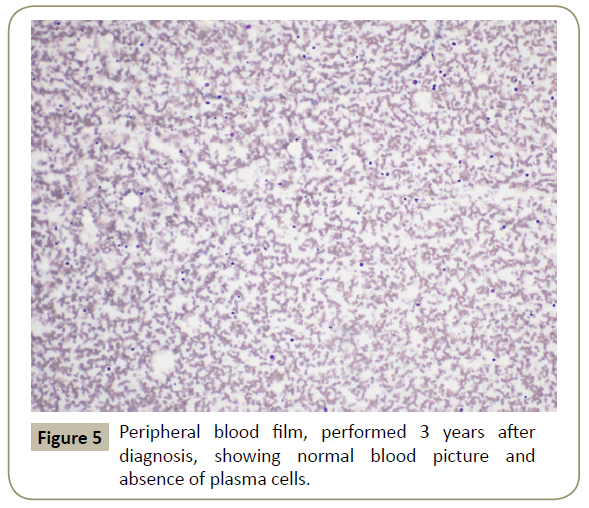
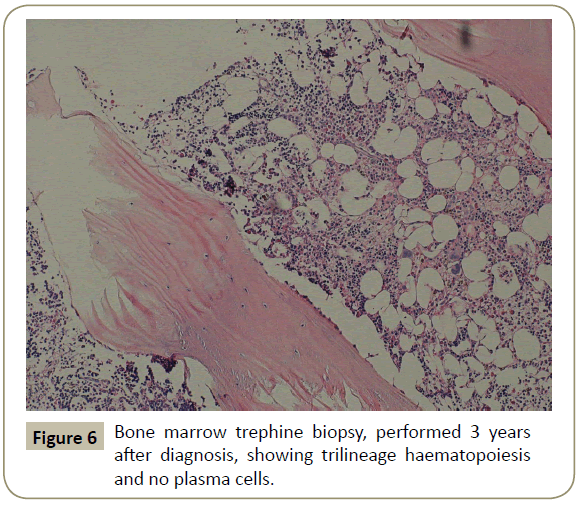
Throughout her follow-up at the HSCT out-patient clinic, the patient remained very well clinically without any major complication. Re-staging evaluation performed 3 years after the diagnosis of her disease showed evidence of continued CR of PCL (Figures 5 and 6). She was last seen at the transplant clinic in early March 2019. She was asymptomatic; her physical examination revealed no abnormality and her laboratory investigations were within normal limits. She was continued on lenalidomide maintenance and acyclovir prophylaxis and she was given a new follow-up appointment.
Discussion
Primary PCL is distinct clinicopathological entity of plasma cell dyscrasias accounting for 60-70% of all PCLs [16]. Primary PCL does not arise from pre-existing MM and is characterized by an aggressive clinical course [16]. It may represent a valid model of genetic and molecular alterations associated with HR-MM [17,18]. Compared to MM, it shows: more complex and heterogeneous molecular patterns, greater number of genomic alterations and higher incidence of: 13q deletion, 17 p deletion and translocation involving immunoglobulin heavy chain locus as well as lower incidence of hyperdiploidy [18]. New high throughout technologies such as next generation sequencing have allowed: a better understanding of the molecular basis, development of risk stratification and provision of insights into targeted therapeutic strategies for primary PCL [17,18].
Early intensive chemotherapy is recommended as the disease is aggressive and due to difficulty in achieving remission [19]. In the past, the only available therapy for patients with primary PCL was conventional cytotoxic chemotherapy which failed to control the disease leading to dramatically poor outcome [16]. During the last few decades, initially autologous HSCT then novel therapies such as immunomodulatory drugs and proteasome inhibitors, particularly bortezomib, have led to improvement in survival of patients with primary PCL [16]. Several studies have shown that bortezomib given in various combinations with other novel agents such as thalidomide and lenalidomide or conventional chemotherapeutic agents such as doxorubicin, cyclophosphamide, melphalan, vincristine and prednisolone or dexamethasone have improved the outcome of patients with primary PCL [15,16,19,20].
In patients with primary PCL, the long-term survival remains poor despite the availability of novel therapies such as proteasome inhibitors and immunomodulatory agents [21]. Induction therapies with various combinations of novel therapies such as VTD or VRD appear to be superior to the historically used alkylating agents in the treatment of primary PCL [22-24]. In patients with primary PCL, overall survival (OS) with novel therapies has extended to 2.85 years compared to 1.16 years in patients treated with traditional chemotherapy such as VAD regimen (vincristine, doxorubicin and dexamethasone) [25]. Also, recent retrospective studies suggest that the incorporation of novel agents as well as autologous HSCT in the management of primary PCL has improved the median OS to > 24 months [2,12,26].
Studies have shown that: (1) induction with bortezomib containing or lenalidomide-based regimens is highly effective in reducing the rate of early mortality, and (2) consolidation with single or tandem autologous HSCT prolong OS [3,15,16,27,28]. HSCT has increased OS and duration of response by 69% and 88% respectively [24]. Despite the improvement in outcome achieved by autologous HSCT, the results of autologous HSCT in primary PCL are still inferior to those of autologous HSCT in MM [26].
In a study that included 147 patients and compared the outcomes of autologous and allogeneic HSCT in patients with primary PCL, the following results were obtained: (1) the 3 year OS rates in patients subjected to autologous versus allogeneic HSCT were 64% and 39% respectively, and (2) the cumulative incidences of relapse after autologous and allogeneic HSCT were 61% and 38% respectively [5]. The authors concluded that although allogeneic HSCT was associated with significantly lower relapse rates, it carried much higher risk of non-relapse mortality (NRM) without OS benefit [5].
After induction phase of treatment, management of patients with primary PCL should include: tandem autologous HSCT, followed by consolidation therapy and maintenance treatment in all eligible patients [29]. In primary PCL patients, development of complex treatment algorithms that combine intensive chemotherapeutic agents such as cyclophosphamide, doxorubicin and dexamethasone and novel therapies such as bortezomib induction followed by HSCT, autologous or allogeneic then maintenance therapy may ultimately be translated into higher response rates and improved survival [7,16,21,27].
After achieving complete remission (CR) with intensive chemotherapy or novel agents, tandem autologous HSCT has been shown to improve OS not only in patients with primary PCL but also in patients with secondary PCL [30,31]. A large prospective trial performed in patients with primary PCL showed that tandem autologous/autologous HSCT followed by maintenance therapy may be superior to tandem autologous/ allogeneic HSCT regarding OS and progression free survival (PFS) [32]. Therefore, a tandem autologous HSCT should always be considered in patients achieving CR after the first autologous HSCT while autologous HSCT followed by reduced intensity conditioning (RIC)-allogeneic HSCT may be considered in selected cases [14,32].
Allogeneic HSCT can be offered to selected cases particularly younger patients without comorbid medical conditions with the potential benefit of graft versus PCL (GVPCL) effect [7,21,29,33]. However, the efficacy of allografts in primary PCL remains unknown due to paucity of prospective trials and the limited number of retrospective studies and case series that report long-term outcome of patients treated with allogeneic HSCT [21]. Prior to allogeneic HSCT in patients with primary PCL, induction therapy with VRD, VAD or hyper-CVAD (cyclophosphamide + VAD) is required to induce CR of the disease [34-36]. In patients with primary PCL, autologous HSCT followed by allogeneic HACT has been found to be superior to upfront allogeneic HSCT due to lower rates of NRM, that is, upfront allografts are associated with worse OS than autologous HSCT alone or autologous HSCT followed by allogeneic HSCT due to high NRM [37]. Haploidentical HSCT can be a potentially curative therapeutic modality in patients with primary PCL who do not have an HLA-identical donor [38]. Additionally, haploidentical HSCT may provide GVPCL effect in patients with primary PCL subjected to allogeneic HSCT [38].
In patients with primary PCL, post-HSCT maintenance therapy has been associated with longer PFS and a trend towards a longer OS [22]. The following agents have been used in maintenance therapy following HSCT in patients with PCL: α-interferon, thalidomide, bortezomib, lenalidomide, and pomalidomide either as single agents or in combination with dexamethasone [21,22,34,35,39,40]. Lenalidomide maintenance in the post-HSCT setting was linked to a GVPCL effect in a single case report, unlike the situation in lenalidomide maintenance following allografting in MM which is not feasible [21].
Treatment of relapsed/refractory primary PCL should include combinations of lenalidomide or pomalidomide + dexamethasone + carfilzomib or monoclonal antibodies such as daratumumab or elotuzumab [29]. Large prospective multicenter studies are required to provide: (1) better understanding of the pathogenesis, (2) revised definitions and risk stratification, and (3) specific treatment algorithms that incorporate novel therapies and cytotoxic chemotherapeutic agents, in induction and maintenance phases of treatment, as well as various forms of HSCT in the management of patients with primary PCL [7,27].
The patient presented received 2 cycles of intensive therapy composed of a combination of cytotoxic chemotherapy in addition to novel agents. The VTD-PACE therapy induced CR of her aggressive primary PCL. The patient was a candidate for allogeneic HSCT, but due to unavailability of sibling donors, she was planned for tandem autologous HSCT followed by consolidation and maintenance therapies in order to control her disease optimally. Fortunately, the line of management adopted for this patient ultimately cured her aggressive PCL.
Conclusion
Ideally, patients with PCL should receive aggressive induction therapy followed by allogeneic HSCT in patients who are eligible for this therapeutic modality. In the absence of sibling donors or in case the patient is not a candidate for stem cell allograft, tandem autologous HSCT followed by consolidation treatment then long-term maintenance therapy looks a very valid alternative therapeutic modality that can produce not only durable disease control but also cure.
Acknowledgement
We are grateful to all medical, nursing and technical staff at KFSH in Dammam, Saudi Arabia who took care of the patient presented. The authors declare that they have no conflict of interest. They also declare that they all participated in taking care of the patient presented and that they participated in preparing and writing the manuscript.
References
- Zatula A, Dikic A, Mulder C, Sharma A, Vågbø CB, et al. (2017) Proteome alterations associated with transformation of multiple myeloma to secondary plasma cell leukemia. Oncotarget 8: 19427-19442.
- Gonsalves WI, Rajkumar SV, Go RS, Dispenzieri A, Gupta V, et al. (2014) Trends in survival of patients with primary plasma cell leukemia: a population-based analysis. Blood 124: 907-912.
- Jung SH, Lee JJ, Kim K, Suh C, Yoon DH, et al. (2017) The role of frontline autologous stem cell transplantation for primary plasma cell leukemia: a retrospective multicenter study (KMM160). Oncotarget 8: 79517-79526.
- Gertz MA, Buadi FK (2010) Plasma cell leukemia. Haematologica 95: 705-707.
- Mahindra A, Kalaycio ME, Vela-Ojeda J, Vesole DH, Zhang MJ, et al. (2012) Hematopoietic cell transplantation for primary plasma cell leukemia: results from the Center for International Blood and Marrow Transplant Research. Leukemia 26: 1091-1097.
- Tiedemann RE, Gonzalez-Paz N, Kyle RA, Santana-Davila R, Price-Troska T, et al. (2008) Genetic aberrations and survival in plasma cell leukemia. Leukemia 22: 1044-1052.
- Fernández de Larrea C, Kyle RA, Durie BG, Ludwig H, Usmani S, et al. (2013) Plasma cell leukemia: consensus statement on diagnostic requirements, response criteria and treatment recommendations by the International Myeloma Working Group. Leukemia 27: 780-791.
- Katodritou E, Terpos E, Kelaidi C, Kotsopoulou M, Delimpasi S, et al. (2014) Treatment with bortezomib-based regimens improves overall response and predicts for survival in patients with primary or secondary plasma cell leukemia: Analysis of the Greek myeloma study group. Am J Hematol 89: 145-150.
- Kar R, Priyadarshini SG, Niraimathi M, Basu D, Badhe BA (2012) Clinico-pathological spectrum of primary plasma cell leukemia diagnosed at a tertiary care centre in South India over 5 year period. Indian J Hematol Blood Transfus 28: 170-174.
- Liedtke M, Medeiros BC (2010) Plasma cell leukemia: concepts and management. Expert Rev Hematol 3: 543-549.
- Granell M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, et al. (2017) Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition. Haematologica 102: 1099-1104.
- Usmani SZ, Nair B, Qu P, Hansen E, Zhang Q, et al. (2012) Primary plasma cell leukemia: clinical and laboratory presentation, gene-expression profiling and clinical outcome with Total Therapy protocols. Leukemia 26: 2398-2405.
- Talamo G, Dolloff NG, Sharma K, Zhu J, Malysz J (2012) Clinical features and outcomes of plasma cell leukemia: a single-institution experience in the era of novel agents. Rare Tumors 4: e39.
- Ravinet A, Bay JO, Tournilhac O (2014) Plasma cell leukemia. Bull Cancer 101: 1048-1058.
- Musto P, Simeon V, Todoerti K, Neri A (2016) Primary plasma cell leukemia: identity card 2016. Curr Treat Options Oncol 17: 19.
- Katodritou E, Terpos E, Delimpasi S, Kotsopoulou M, Michalis E, et al. (2018) Real-world data on prognosis and outcome of primary plasma cell leukemia in the era of novel agents: a multicenter national study by the Greek Myeloma Study Group. Blood Cancer J 8: 31.
- Simeon V, Todoerti K, La Rocca F, Caivano A, Trino S, et al. (2015) Molecular classification and pharmacogenetics of primary plasma cell leukemia: an initial approach toward precision medicine. Int J Mol Sci 16: 17514-17534.
- Cifola I, Lionetti M, Pinatel E, Todoerti K, Mangano E, et al. (2015) Whole-exome sequencing of primary plasma cell leukemia discloses heterogeneous mutational patterns. Oncotarget 6: 17543-1758.
- Tamura S, Koyama A, Shiotani C, Kurihara T, Nishikawa A, et al. (2014) Successful bortezomib/ dexamethasone induction therapy with lenalidomide in an elderly patient with primary plasma cell leukemia complicated by renal failure and pulmonary hypertension.
- D'Arena G, Valentini CG, Pietrantuono G, Guariglia R, Martorelli MC, et al. (2012) Frontline chemotherapy with bortezomib-containing combinations improves response rate and survival in primary plasma cell leukemia: a retrospective study from GIMEMA Multiple Myeloma Working Party. Ann Oncol 23: 1499-1502.
- Nishihori T, Abu Kar SM, Baz R, Alsina M, Harousseau JL, et al. (2013) Therapeutic advances in the treatment of primary plasma cell leukemia: a focus on hematopoietic cell transplantation. Biol Blood Marrow Transplant 19: 1144-1151.
- Gowda L, Shah MV, Badar I, Bashir Q, Shah N, et al. (2018) Primary plasma cell leukemia: stem cell transplant in an era of novel induction drugs. Biol Blood Marrow Tranplant 24: S260–S261.
- Ueda S, Kubo M, Matsuura N, Matsunaga H, Kataoka S, et al. (2013) Stringent complete remission of primary plasma cell leukemia with reduced-dose bortezomib, lenalidomide and dexamethasone: a case report and review of the literature. Intern Med 52: 1235-1238.
- Pagano L, Valentini CG, De Stefano V, Venditti A, Visani G, et al. (2011) Primary plasma cell leukemia: a retrospective multicenter study of 73 patients. Ann Oncol 22: 1628-35.
- Iriuchishima H, Ozaki S, Konishi J, Matsumoto M, Murayama K, et al. (2016) Primary plasma cell leukemia in the era of novel agents: a multicenter study of the Japanese Society of Myeloma. Acta Haematol 135: 113-121.
- Drake MB, Iacobelli S, van Biezen A, Morris C, Apperley JF, et al. European Group for Blood and Marrow Transplantation and the European Leukemia Net (2010) Primary plasma cell leukemia and autologous stem cell transplantation. Haematologica 95: 804-809.
- Royer B, Minvielle S, Diouf M, Roussel M, Karlin L, et al. (2016) Bortezomib, doxorubicin, cyclophosphamide, dexamethasone induction followed by stem cell transplantation for primary plasma cell leukemia: a prospective phase II study of the Intergroupe Francophone du Myélome. J Clin Oncol 34: 2125-2132.
- Wang H, Li JY, Sun C, Zhou X (2017) Advances in the diagnosis and therapy of primary plasma cell leukemia -review. Zhongguo Shi Yan Xue Ye Xue Za Zhi 25: 1837-1841.
- Gavriatopoulou M, Musto P, Caers J, Merlini G, Kastritis E, et al. (2018) European myeloma network recommendations on diagnosis and management of patients with rare plasma cell dyscrasias. Leukemia 32: 1883-1898.
- Iino M, Nakajima A, Shindo H (2008) Long-term complete remission in primary plasma cell leukemia after treatment with VAD chemotherapy and tandem autologous peripheral blood stem cell transplantation. Gan To Kagaku Ryoho 35: 2441-2443.
- Sekiguchi Y, Shimada A, Wakabayashi M, Sugimoto K, Tomita S, et al. (2014) A case of secondary plasma cell leukemia resistant to novel agents, in which stringent complete remission was achieved and maintained for a long period of time after VAD therapy and tandem autologous transplantation. Int J Clin Exp Pathol 7: 6313-6122.
- Royer B, Diouf M, Roussel M, Karlin L, Hulin C, et al. (2016) Long term follow-up of hematopoietic stem cell transplantation (HSCT) for primary plasma cell leukemia (pPCL): final results of a prospective study of IFM Group. Blood 128: 4612.
- Neri A, Todoerti K, Lionetti M, Simeon V, Barbieri M, et al. (2016) Primary plasma cell leukemia 2.0: advances in biology and clinical management. Expert Rev Hematol 9: 1063-1073.
- Hosono N, Yamauchi T, Nakamura T, Yamashita T, Ueda T (2008) Plasma cell leukemia induced to complete remission with negative minimal residual disease by qualitative PCR analysis after hyper-CVAD and high-dose melphalan followed by autologous peripheral blood stem cell transplantation. Gan To Kagaku Ryoho 35: 533-537.
- Yamashita Y, Tamura S, Oiwa T, Kobata H, Kuriyama K, et al. (2017) Successful intrathecal chemotherapy combined with radiotherapy followed by pomalidomide and low-dose dexamethasone maintenance therapy for a primary plasma cell leukemia patient. Hematol Rep 9: 6986.
- Saccaro S, Fonseca R, Veillon DM, Cotelingam J, Nordberg ML, et al. (2005) Primary plasma cell leukemia: report of 17 new cases treated with autologous or allogeneic stem-cell transplantation and review of the literature. Am J Hematol 78: 288-294.
- Lawless S, Simona I, van Biezen A, Koster L, Chevallier P, et al. (2016) Comparison of haematopoietic stem cell transplantation approaches in primary plasma cell leukaemia. Blood 128: 2293.
- Nonami A, Miyamoto T, Kuroiwa M, Kunisaki Y, Kamezaki K, et al. (2007) Successful treatment of primary plasma cell leukaemia by allogeneic stem cell transplantation from haploidentical sibling. Jpn J Clin Oncol 37: 969-972.
- Mak YK, Chan CH, Chen YT, Lau SM, So CC, et al. (2003) Consolidation therapy with autologous blood stem cell transplantation in a patient with primary plasma cell leukaemia. Clin Lab Haematol 25: 55-58.
- Abe M, Yokoyama H, Tohmiya Y, Okitsu Y, Ohguchi H, et al. (2009) Plasma cell leukemia maintaining complete remission by syngeneic stem cell transplantation combined with low-dose thalidomide maintenance therapy. Intern Med 48: 1833-1835.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences