Clinical Strategies for PARP Inhibitors beyond Homologous Recombination Deficiency
Science for Life Laboratory, Division of Translational Medicine and Chemical Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden
- *Corresponding Author:
- Thomas Helleday
Department of Medical Biochemistry and Biophysics
Karolinska Institutet, Stockholm, Sweden.
Tel: +46 8 52480000
E-mail: thomas.helleday@scilifelab.se
Received date: October 25, 2017; Accepted date: October 25, 2017; Published date: November 01, 2017
Citation: Helleday T (2017) Clinical Strategies for PARP Inhibitors beyond Homologous Recombination Deficiency. J Med Oncol. Vol.1 No.1:2
Abstract
recurrent ovarian cancer and today there are already three PARP inhibitors approved; olaparib, rucaparib and niraparib. PARP inhibitors selectively target cancers with mutation of BRCA1 or BRCA2 alleles owing to their homologous recombination deficiency (HRD).
Introduction
PARP inhibitors represent a new paradigm in the treatment of recurrent ovarian cancer and today there are already three PARP inhibitors approved; olaparib, rucaparib and niraparib. PARP inhibitors selectively target cancers with mutation of BRCA1 or BRCA2 alleles owing to their homologous recombination deficiency (HRD) [1,2]. The synthetic lethal relationship between PARP and HRD makes these inhibitors highly effective to the cancer cells while being tolerable and with few adverse effects [3]. Recent approval also includes non-BRCA mutant ovarian cancer, which may come as a surprise. The reason is that HRD in cancer can occur by mutations in genes other than in BRCA1 or BRCA2, which are also critical for homologous recombination, such as ATM, FANCD2, FANCA, FANCF or FANCC [4-6]. As there are >100 genes encoding proteins involved in homologous recombination (HR), it is not a trivial matter to identify which mutations may contribute to PARP inhibitor sensitivity. To face this challenge there has been extensive HRD biomarker development by either functional assays scoring for RAD51 foci [7] or by exploiting the fact that the HRD gives a unique genomic scarring that can be detected using mutational signatures to develop a specific HRD signature [8] . To date the most advanced HRD signature, called HRDetect, exploits a mutation substitution signature 3, rearrangement signatures and insertion or deletions [9]. Combining all this information together, it can with high accuracy predict defects in HR. Since PARP inhibitors have been demonstrated in clinical trials to be potentially effective treatment also in BRCA mutated breast, pancreatic, and prostate cancers [10] one may use HRD signatures to predict whether also these cancers may respond to PARP inhibitor treatment in monotherapy. While there is convincing evidence of PARP inhibitors targeting HRD cancers, current research also indicate that it is possible to extend the use of PARP inhibitors beyond HRD.
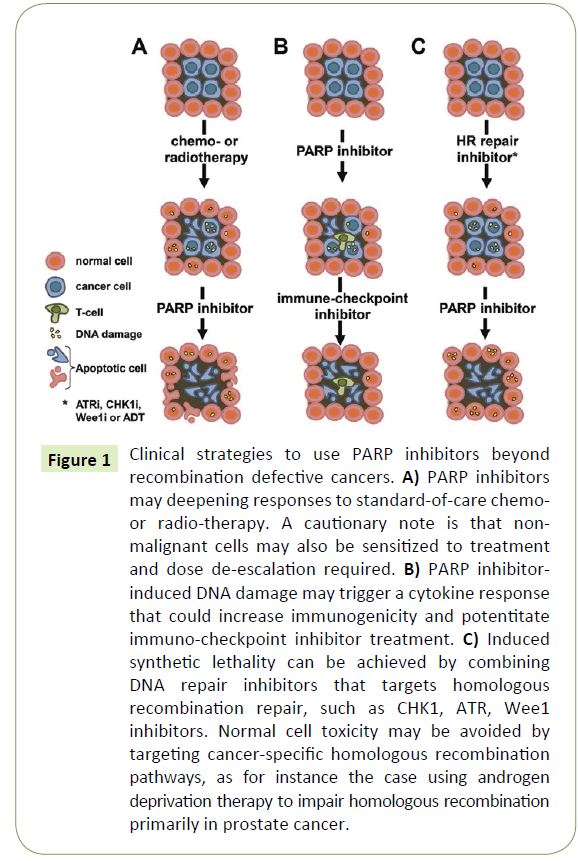
The original rationale to develop PARP inhibitors as anti-cancer treatments was to potentiate DNA damaging chemotherapy drugs, such as temozolomide, by inhibiting DNA repair [11- 13]. While this is likely to increase the anti-cancer effect, it will also increase the toxicity of the drug (Figure 1A). Hence, such studies have been carried out with great caution to determine any putative safety issues. Indeed, the combination of PARP inhibitors and chemotherapy is reported to be tolerable in many cases and numerous combinations have been tested in the clinic, e.g. with irinotecan, gemcitabine, carbo- or cisplatin, paclitaxel, dacarbacine and liposomal doxorubicin. Although some individual patients may benefit tremendously from such a combination strategy, it does not seem to be the case for all patients and obtaining a significant increase in overall survival in certain cancer populations may be prove to be difficult. Also, the clinical trial designs of these types of combinations appear too often to be based primarily on clinical feasibility rather than the basic science rationale. However, given the large amount of ongoing studies some are likely going to provide sufficient benefit to patients overall to obtain regulatory approvals.
Figure 1: Clinical strategies to use PARP inhibitors beyond recombination defective cancers. A) PARP inhibitors may deepening responses to standard-of-care chemoor radio-therapy. A cautionary note is that nonmalignant cells may also be sensitized to treatment and dose de-escalation required. B) PARP inhibitorinduced DNA damage may trigger a cytokine response that could increase immunogenicity and potentitate immuno-checkpoint inhibitor treatment. C) Induced synthetic lethality can be achieved by combining DNA repair inhibitors that targets homologous recombination repair, such as CHK1, ATR, Wee1 inhibitors. Normal cell toxicity may be avoided by targeting cancer-specific homologous recombination pathways, as for instance the case using androgen deprivation therapy to impair homologous recombination primarily in prostate cancer.
A potential amenable approach is the combination of PARP inhibitors with radiotherapy. That is because the DNA damage can be restricted to the cancer area and may to a lesser extent potentiate normal tissue toxicity. It has been shown that PARP inhibitors potentiate radiation-induced cytotoxicity primarily in replicating cells and hence the PARP inhibitors may be particularly useful to radiosensitize for example glioblastomas, since replication is very low in normal brain tissue [14,15]. Currently, such strategy is tested in clinical trials (NCT01514201).
The introduction of immune checkpoint oncology drugs is fundamentally changing clinical practise and since DNA damaging drugs also causes a cytokine response it is likely that a combination may be beneficial. Since PARP inhibitors cause DNA damage in HRD cancer cells one would expect that this may also cause an immune response that can be exploited using immunecheckpoint inhibitors (Figure 1B). Indeed, CTLA-4 blockade has been shown to improve the effect of PARP inhibitors in BRCA1 deficient ovarian cancer [16] and such combination is currently being evaluated in the clinic (NCT02571725, NCT02953457). The rationale could be that a partial response provided by a PARP inhibitor may, in combination with an immune-checkpoint blockade inhibitor, provide a deeper long lasting response.
An interesting question is if the effect of a PARP-immuneoncology drug combination is limited to HRD cancers, or if a larger patient cohort could also be targeted with the combination? One report suggests PD-L1 expression is triggered by PARP inhibitor treatment and may hence limit the anti-cancer response to PARP inhibitors [17]. The authors of this report argue a combination may potentially improve efficacy, which then could be independent of HRD status.
PARP has also a role in transcription that is distinct to its role in DNA repair. It has for instance been demonstrated that hormone-induced transcription in breast cancer is mediated by PARP. In this case, the PAR polymers generated by PARP are converted, by PARG and NUDT5, into ATP to drive transcription of hormone-dependent genes [18]. This seems not to be specific to breast cancer, as it is reported that androgen receptorinduced transcription is also PARP dependent and targeted by PARP inhibitors [19]. Thus, PARP inhibitors may in certain context affect transcription, in for instance hormone-dependent cancers. This may in turn affect the responses to immune-oncology drugs that would be independent of HRD in cancer.
One of the most interesting concepts of combining PARP inhibitors is to combine them with targeted agents that generate HRD to create an induced synthetic lethality (Figure 1C). In this concept a HRD is created in a cancer using a targeted treatment, which then can be exploited with a PARP inhibitor. HRD can be achieved by ATR, CHK1 and Wee1 inhibitors, as the ATR and CHK1 kinases are critically important in HR signalling [20] and forced Cdk1 activity by Wee1 inhibition impairs HR [21]. Furthermore, the combination of a CHK1 and PARP inhibitor has been reported to act synergistically in pre-clinical cancer models [22]. Excitingly, PARP inhibitor combination trials are currently ongoing or planned with ATR and Wee1 inhibitors (NCT02511795, NCT02576444). A general caution is that the ATR/CHK1/Wee1 inhibition would likely impair HR regardless of the cell type, which may also augment adverse effects by PARP inhibitors.
One way to avoid increased toxicity in the induced synthetic lethality approach would be to selectively induce a HRD in the cancer cells and not in all cells. For this to work one has to identify a cancer-specific HR pathway. Interestingly, it has been demonstrated that DNA repair is regulated by the androgen receptor in primary prostate cancer [23-26] and that androgen deprivation therapy synergize with PARP inhibitors [24]. The combination treatment with PARP inhibitor and androgen deprivation therapy is likely to be well tolerated as the toxicity is limited to the androgen responsive tissue, e.g. the prostate gland. Currently, a small phase I study is carried out combining Olaparib and Degarelix to monitor PARP inhibition (NCT02324998).
Conclusion
In conclusion, while PARP inhibitors are making way to transform treatment of HRD cancers, there are also other opportunities for PARP inhibitors to be more widely useful in treatment of cancer, by (1) combining them with standard-of-care chemotherapy, (2) combining them with immune-checkpoint inhibitors or (3) in combination with targeted therapy that impairs HR, preferentially to induce a cancer-specific HRD. Exciting times lay ahead for PARP inhibitors once reports from the many ongoing clinical trials will become available.
Funding
The laboratory is primarily funded by the Swedish Research Council, the European Research Council, Swedish Cancer Society, the Swedish Children’s Cancer Foundation, the Strategic Research Foundation and the Swedish Pain Relief Foundation.
COI Statement
The author is inventor of a patent to use PARP inhibitors in homologous recombination defective cancers and receives royalty from PARP inhibitor sales.
References
- Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, et al. (2005) Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose)polymerase. Nature 434: 913-917.
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, et al. (2005) Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434: 917-921.
- Fong PC, Boss DS, Yap TA, Tutt A, Wu P, et al. (2009) Inhibition of poly(ADP-Ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361: 123-134.
- Bryant HE, Helleday T (2006) Inhibition of poly (ADP-ribose) polymerase activates ATM which is required for subsequent homologous recombination repair. Nucleic Acids Res 34: 1685-1691.
- McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, et al. (2006) Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-Ribose) polymerase inhibition. Cancer Res 66: 8109-8115.
- Taniguchi T, Tischkowitz M, Ameziane N, Hodgson SV, Mathew CG, et al. (2003) Disruption of the Fanconi anemia-BRCA pathway in cisplatin-sensitive ovarian tumors. Nat Med 9: 568-574.
- Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, et al. (2010) Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res 16: 2344-2351.
- Helleday T, Eshtad S, Nik-Zainal S (2014) Mechanisms underlying mutational signatures in human cancers. Nat Rev Genet 15: 585-598.
- Davies H, Glodzik D, Morganella S, Yates LR, Staaf J, et al. (2017) HRDetect is a predictor of BRCA1 and BRCA2 deficiency based on mutational signatures. Nat Med 23: 517-525.
- Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, et al. (2015) Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol 33: 244-250.
- Curtin NJ, Wang LZ, Yiakouvaki A, Kyle S, Arris CA, et al. (2004) Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res 10: 881-889.
- Delaney CA, Wang LZ, Kyle S, White AW, Calvert AH, et al. (2000) Potentiation of temozolomide and topotecan growth inhibition and cytotoxicity by novel poly(adenosine diphosphoribose) polymerase inhibitors in a panel of human tumor cell lines. Clin Cancer Res 6: 2860-2867.
- Plummer R, Jones C, Middleton M, Wilson R, Evans J, et al. (2008) Phase I study of the poly(ADP-ribose) polymerase inhibitor, AG014699, in combination with temozolomide in patients with advanced solid tumors. Clin Cancer Res 14: 7917-7923.
- Chalmers AJ, Lakshman M, Chan N, Bristow RG (2010) Poly(ADP-ribose) polymerase inhibition as a model for synthetic lethality in developing radiation oncology targets. Semin Radiat Oncol 20: 274-281.
- Loser DA, Shibata A, Shibata AK, Woodbine LJ, Jeggo PA, et al. (2010) Sensitization to radiation and alkylating agents by inhibitors of poly(ADP-ribose) polymerase is enhanced in cells deficient in DNA double-strand break repair. Mol Cancer Ther 9: 1775-1787.
- Higuchi T, Flies DB, Marjon NA, Mantia-Smaldone G, Ronner L, et al. (2015) CTLA-4 blockade synergizes therapeutically with PARP inhibition in BRCA1-deficient ovarian cancer. Cancer Immunol Res 3: 1257-1268.
- Jiao S, Xia W, Yamaguchi H, Wei Y, Chen MK, et al. (2017) PARP inhibitor upregulates PD-L1 expression and enhances cancer-associated immunosuppression. Clin Cancer Res 23: 3711-3720.
- Wright RH, Lioutas A, Le Dily F, Soronellas D, Pohl A, et al. (2016) ADP-ribose-derived nuclear ATP synthesis by NUDIX5 is required for chromatin remodeling. Science 352: 1221-1225.
- Schiewer MJ, Goodwin JF, Han S, Brenner JC, Augello MA, et al. (2012) Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2: 1134-1149.
- Sorensen CS, Hansen LT, Dziegielewski J, Syljuasen RG, Lundin C, et al. (2005) The cell-cycle checkpoint kinase Chk1 is required for mammalian homologous recombination repair. Nat Cell Biol 7: 195-201.
- Krajewska M, Heijink AM, Bisselink YJ, Seinstra RI, Sillje HH, et al. (2013) Forced activation of Cdk1 via wee1 inhibition impairs homologous recombination. Oncogene 32: 3001-3008.
- Tang Y, Hamed HA, Poklepovic A, Dai Y, Grant S, et al. (2012) Poly(ADP-ribose) polymerase 1 modulates the lethality of CHK1 inhibitors in mammary tumors. Mol Pharmacol 82: 322-332.
- Al-Ubaidi FL, Schultz N, Egevad L, Granfors T, Loseva O, et al.(2013) Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res 19:1547-1556.
- Asim M, Tarish F, Zecchini HI, Sanjiv K, Gelali E, et al. (2017) Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun 8: 374.
- Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, et al. (2013) Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov 3: 1245-1253.
- Tarish FL, Schultz N, Tanoglidi A, Hamberg H, Letocha H, et al. (2015) Castration radiosensitizes prostate cancer tissue by impairing DNA double-strand break repair. Sci Transl Med 7: 312re311.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences