Brain DC Potentials Evoked by Listening to MozartÃÆâÃâââ¬Ãâââ¢s Sonata K. 448, AlbinoniÃÆâÃâââ¬Ãâââ¢s Adagio, SchubertÃÆâÃâââ¬Ãâââ¢s Fantasia, and Brown Noise: Indications of a Mozart Effect Independent of Mood and Arousal
1 Department of Basic Psychological Research Methods, Vienna, Austria
2 Department of Environmental Health, Vienna Austria
- *Corresponding Author:
- Michael Trimmel
Department of Basic Psychological Research Methods, Vienna, Austria.
Tel: +4314016034901
E-mail: michael.trimmel@meduniwien.ac.at
Received date: January 28, 2017; Accepted date: February 06, 2017; Published date: February 14, 2017
Citation: Trimmel M, Goger C, Spitzer U, et al. Brain DC Potentials Evoked by Listening to Mozart’s Sonata K. 448, Albinoni’s Adagio, Schubert’s Fantasia, and Brown Noise: Indications of a Mozart Effect Independent of Mood and Arousal. J Psychol Brain Stud. 2017, 1:2.
Copyright: © 2017 Trimmel M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Context: The Mozart effect describes that after listening to Mozart’s sonata K. 448 spatial reasoning was superior. Up to now there is puzzling evidence on the validity of this effect and controversial explanations are offered.
Objective: Based on the findings which indicate that Mozart’s sonata activates brain circuits related to attention and cognitive processing, changes in brain DC potentials induced by Mozart’s sonata and three control conditions were investigated.
Design: According to a repeated ANOVA design brain DC potential changes, autonomic arousal, and mood were compared in four conditions of stimulation: Mozart’s Sonata K. 448, Albinoni’s Adagio, Schubert’s Fantasia, and brown noise.
Setting and Interventions: Participants listened to three pieces of music and to brown noise which were presented in a balanced order while electrophysiological signals were recorded (electroencephalogram, heart rate, and skin conductance). After 4 min of stimulation ratings of mood were initiated. Participants: A total of 38 (19 females) non-musicians participated in the study.
Main outcome measures: For stimulation with Mozart prefrontal and frontal brain potentials shifted negatively, whereas for all other stimulations positively. No relevant differences of autonomic arousal were observed. Mood ratings were comparable for musical pieces and negative for brown noise.
Results: Negative brain DC potentials, most pronounced at prefrontal sites in the Mozart condition, only indicate an attention-related activation which primes spatial information processing.
Conclusions: Results support the Mozart effect independent of mood and arousal. The specific stimulation of two different but similar musical streams is offered as a new explanation of the Mozart effect.
Keywords
Electroencephalography; Brain waves; Music; Mood; Arousal; Attention
Introduction
Rauscher et al. [1] reported a superior performance in spatial reasoning after listening to Mozart’s Sonata for Two Pianos in D-Major, K. 448. This was an interesting finding for practical applications and a compelling message, in particular to discover its psychological and physiological basis, which is still unsolved, although having been investigated by numerous studies and methods including neurophysiological approaches.
The initial findings [1] are still under discussion, as on the one hand, they were successfully replicated [2-9], and on the other hand, there were also attempts which were unable to replicate the so called Mozart effect [10-12]. As one reason for the confusing observations methodological reasons like between subjects and within subject designs [11,13], besides other methodological shortcomings and the validity in particular were discussed [13]. Another reason of contradictory results comes from a misconception that listening to Mozart enhances intelligence, this was not claimed; the Mozart effect was described for spatialtemporal tasks involving mental imagery and temporal ordering [1,14].
The sustained or even growing interest on the functional properties of Mozart’s sonata K. 448 is motivated by the application in diverse areas of applications, which were successful in the treatment of epilepsy in children [15-20] and adults [20] as well as in Long Evans rats [17], and there are also positive effects, like in geriatric patients with mild cognitive impairment in which the spatial-temporal performance remains to be constant in time [21], in patients suffering from Alzheimer’s disease, where spatial-temporal reasoning improved [22], and also a better acquisition of laparoscopic skills [23]. However, there are also reports with no significant effects caused by the Sonata K. 448, like in the adenoma detection rate in endoscopists by comparing K.488 with music from four other genres [24], or on recognition of upright or rotated Chinese characters during listening to K.448 [25], on the reliability of the Humphrey visual field test in persons with glaucoma [26], thus indicating that the Mozart effect seems not to be a universal method to enhance cognitive performance, although it seems that the Mozart effect may extend beyond cognitive tasks and includes sensorimotor adaptation for strategic movement control but not for adaptive recalibration [27].
In addition to the puzzling evidence on performance effects of the sonata K. 448, various interpretations of the fundamental functional mechanisms were offered as explanations as well. The first framework [1,2] was the trion model [28], a structured mathematical model based (and extended) on the organizational principle in which cortical columns are the basic neural networks of the cortex [29] and comprised of subunit minicolumns, the idealized trions. Proposing that music is a prelanguage, it was suggested that firing patterns initiated by music may improve other higher brain functions [2].
In the following, (radical) alternative interpretations were also proposed, as the claim that compelling evidence suggests that the Mozart effect is an artifact of arousal and mood [4]. Indeed, based on activation theory and the proposed relationship of arousal and performance [30], changes in arousal caused by music may appear as effects caused by the intervening variable arousal. The importance to control studies on the Mozart effect for arousal and mood was pointed out shortly after the first publications in that topic [31], in particular as response to early replications comparing the effect of Mozart’s Sonata K. 448 to relaxation or silence [32,33]. As there is evidence that mood enhancing music can increase working memory performance [34], it is worth to notice that there is also empirical evidence that the Mozart effect is not (necessarily) associated with changes in mood [7]. However, when comparing listening to Mozart’s sonata K. 448 with listening to the – sad – Adagio in g-minor by Albinoni, an affective working memory effect was suggested for the observed effect [8].
To investigate which brain mechanisms might be responsible for the Mozart effect, various neurophysiological investigations were conducted. Early attempts in analyzing neurophysiological indications of brain activity of persons stimulated with Mozart’s Sonata K. 448 were already done by means of the electroencephalogram (EEG) to understand the performance in a spatial task [35]. There is convincing evidence of event-related desynchronization in the alpha band and enhanced gamma activity in the EEG. More precisely, when comparing the Sonata K. 448 with Brahms’ Hungarian Dance No. 5 and Haydn’s symphony No. 94, results indicate an event-related desynchronisation in the lower EEG alpha band [36]. Also after listening to the sonata K. 448 in healthy young and elderly adults, contrary to listening to Beethoven’s piano piece “Für Elise”, changes in the EEG alpha band were observed [37]. But an enhanced EEG gamma activity was observed as well, and it was concluded that listening to the Sonata K. 448 facilitates neurophysiological binding of sensory stimuli to a perceived whole in perception [38]. It was concluded that changes in the EEG might be interpreted that listening to Mozart’s sonata K. 448 is activating relevant brain areas, and that may enhance the learning of spatio-temporal rotation tasks [39]. That interpretation is in high concordance with a study indicating that rats, after a long term exposure to the Sonata K. 448, displayed a better performance in maze learning than rats exposed to Beethoven’s “Für Elise” [5]. A recent study confirmed this finding and further explores the underlying mechanisms: the exposure of developing rats to Mozart’s Sonata K. 448 enhanced learning performance in the water maze test; that was accompanied by an enhanced brain-derived neurotropic factor (BDNF) expression level in the dorsal hippocampus, suggesting that a spatial memory improvement may be associated with the enhanced BDNF/TrkB level of dCA3 and dDG [9].
These results are also in line with an early fMRI study [40] indicating that the Sonata K. 448 lead to an activation of networks important for spatial reasoning, contrary to Beethoven’s “Für Elise” and 1930s piano music [40]. Such changes, together with the observed changes in the EEG bands [36,37] and in EEG coherence measures [36], are interpreted as an expression of induced attention processes [36] or as an activation of neuronal cortical circuits related to attentive and cognitive functions [37].
As brain DC potentials are a neurophysiological measure of activation processes [41-48] which reflect attention-related activation [49], they were chosen as the dependent variable to assess the stimulation by Mozart’s Sonata K. 448. To investigate the specificity of this stimulation, three control conditions were applied and measures of subjective experience as well as physiological measures of autonomic arousal [50,51] were used to control for mood and arousal. It was expected that Mozart’s sonata K. 448 would display attention-related cortical activation.
Methods
Subjects
Due to the observation that musicians differ in strategies of music perception [52] and furthermore, that the Mozart effect is rather demonstrable in non-musicians [53] only, thirty-eight right-handed non-musicians without hearing devices and normal or corrected to normal vision participated voluntarily and unpaid in the laboratory experiment. The age of the 19 males and 19 females ranged from 21 to 62 years, with an average of 30 years. Because of storage problems, the data of one person could not be included in the analysis.
Ethics
Written informed consent was obtained from all participants and the study protocol confirms to the ethical guidelines of the “World Medical Association Declaration of Helsinki – Ethical Principles for Medical Research Involving Human Subjects” adopted by the 18th WMA General Assembly, Helsinki, Finland, June 1964 and amended by the 59th WMA General Assembly, Seoul, South Korea, October 2008 as approved by the institutional committee of the University Vienna, Vienna, Austria.
Experimental design
Brain DC potential shifts were investigated according to a 4 (Conditions: Mozart’s Sonata K. 448, Albinoni’s Adagio in g-minor, Schubert’s Fantasia D. 940, and brown noise) × 4 (Time of stimulation in minutes) × 8 (Recording location: Fp1, Fp2, F3, F4, C3, C4, P3, P4) repeated measures ANOVA. Ratings of mood and scores of autonomous activation (heart rate and skin conductance) were investigated according to a repeated measures ANOVA design with 4 Conditions (Mozart’s Sonata K. 448, Albinoni’s Adagio in g-minor, Schubert’s Fantasia D. 940, and brown noise).
Tasks
Four acoustical stimulations (Mozart’s Sonata K. 448, Albinoni’s Adagio in g-minor, Schubert’s Fantasia D. 940, and brown noise) were presented, of each of those 4 minutes were analyzed. The order of the presentation was balanced across test persons to avoid position effects, sequence effects, and cross-over effects. The presentations were separated by a break of 3 minutes, of which the 30 seconds before starting the stimulation served as baseline.
The acoustical stimulation in detail: Mozart’s sonata K.448 for two pianos (D-major; 1st movement, allegro con spirito; interpreted by Murray Perahia and Radu Lupu). Albinoni’s Adagio for organ and strings in g-minor (solist Wolfgang Meyer, conductor Herbert von Karajan of the Berlin Philharmonics) was chosen because an earlier investigation [4] reported that Albinoni’s music would lead to a sad mood with a reduced arousal and that was to be compared to Mozart’s Sonata K. 448. Schubert’s Fantasia for piano duet, D. 940, in f-minor (interpreted by Murray Perahia and Radu Lupu) was selected because an earlier investigation [54] found a comparable mood and level of activation to that of Mozart’s sonata K. 448. Silence served as a control condition in a number of studies about the Mozart effect [1,2,4,12,54], but such a condition seemed uncontrollable because of background noise caused by PCs in the laboratory. The audio materials had been transformed to wave files (44.1 kHz, 16 bit) and were presented in stereo by loudspeakers. The acoustical excerpts had been normalised, so that the maximum peak of the music pieces (Mozart, Albinoni, and Schubert) was at 70 dBA and the maximum peak of brown noise was at 50 dBA. The energy equivalent sound pressure level (LEQ) was 63.3 dBA for Mozart’s sonata, 66.6 dBA for Albinoni’s Adagio, 60.1 dBA for Schubert’s Fantasia and 49.2 dBA for brown noise.
Subjects listened passively four minutes of the acoustical stimulations and did the ratings thereafter, while the stimulation continued.
In each condition, three dimensions of experienced mood – good mood versus bad mood, alertness versus tiredness, and calmness versus agitation – were assessed by the mood questionnaire “Mehrdimensionaler Befindlichkeitsfragebogen” (multidimensional mood questionnaire, MDBF) [55].
Recording of autonomous arousal
Parameters of autonomous arousal were recorded by a Holter recording device (Physiologger; med-Natic GmbH, München, Germany). In order to record skin conductance electrodes were attached on the palm of the person’s non-dominant hand. Heart rate was recorded by electrocardiogram with a modified Lead II configuration according to Einthoven [56].
EEG recordings
The EEG was recorded by a 32-channel DC amplifier (TBZ, Ing. Zickler Ges.m.b.H., model 2320) with high baseline stability and an input impedance ≥ 100 GΩ in the range from DC to 30 Hz [57] and signals were sampled with 62.5 Hz. After careful cleaning and sterilization of recording sites and skin puncturing [58] electrodes were attached to the subjects’ scalps with collodion, according to the 10-20 system [59] at least 1.5 hours before the recording [41] in order to avoid electrode artifacts caused by adjustment processes of the electrode-electrolyte-skin interfaces. A noncephalic sternovertebral reference of two electrodes from skin punctured locations, one at the 7th cervical vertebra and the other on the right sternoclavicular junction with a 5 kΩ potentiometer connecting these two electrodes, individually adjusted, to minimise electrocardiographic components in the EEG, was used [60].
EEG analysis
To analyse brain DC potential changes the EEG data were sampled down by calculating median values for epochs of 1 second. The mean amplitude of the 30 second lasting pre-stimulus period served as baseline. Mean values for each of the four minutes of stimulation had been calculated for the statistical analysis. Brain DC potentials were topographically mapped for each minute of exposure using the spherical spline interpolation algorithm [61,62].
Results
Mood
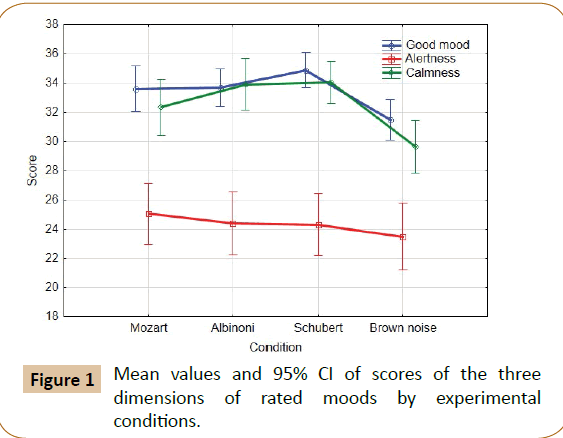
A 4 (Condition) × 3 (Scale: good mood, alertness, and calmness) ANOVA for repeated measures showed a significant effect for condition (F(3,108)=7.65; p=0.000; GGe=0.928) and for scale (F(2,72)=84.04; P=0.00; GGe=0.631). Mean values and 95% CI Figure 1 indicate no significant differences for the scale calmness and for good mood and alertness no differences were observed for musical conditions by 95% CI, but brown noise was experienced different than stimulation by music.
Autonomous activation
A 4 (Condition) × 3 (Time) repeated ANOVA for heart rate revealed only a significant effect for Time (F(3,108)=34.77; p=0.000; GGe=0.764) indicating a condition independent heart rate fluctuation with a higher heart rate in the fifth minute that in the first and third minute and no differences between the first three minutes. Neither the main effect of Condition (F(3,108)=0.001; p=0.998; GGe=0.923) nor the interaction of Condition X Time (F9,324)=1.29; p=0.274; GGe=0.456) reached statistical significance.
A 4 (Condition) × 3 (Time) repeated ANOVA for skin conductance revealed a significant effect for Time (F(3,108)=21.17; p=0.000; GGe=0.606) indicating a higher conductance in the fourth minute than in the first minute and no other significant differences. Neither Condition (F(3,108)=0.852; p=0.446; GGe=0.606) nor the interaction of Condition × Time (F(9,324)=0.668; p=0.654; GGe=0.579) reached statistical significance.
Brain DC potential shifts
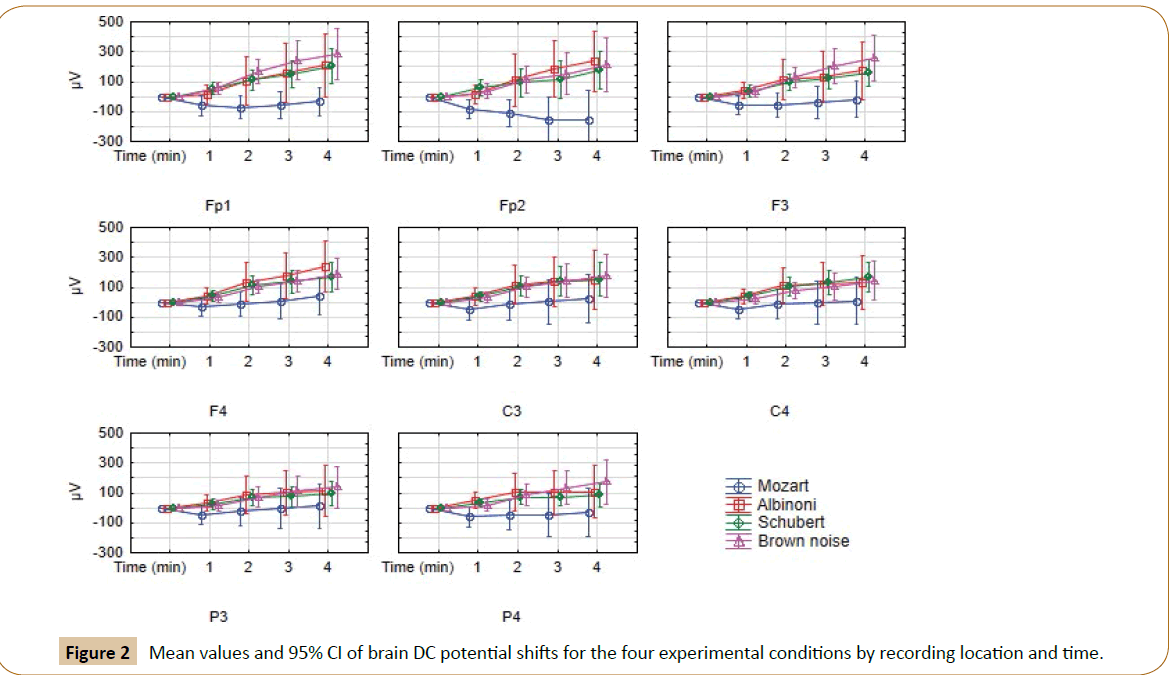
DC potential shifts had been analysed by a 4 (Condition) × 8 (Location: Fp1, Fp2, F3, F4, C3, C4, P3, P4) X 4 (Time: 4 epochs of 1 minute) ANOVA for repeated measures. The analysis revealed a significant 3-way interaction of Condition × Location × Time (F(84,3108)=2.39; P=0.029; GGe=0.070) accompanied with a significant interaction of Condition X Location (F(21,777)=2.73; p=0.019); GGe=0.245) and a main effect for Time (F(4,148)=9.14; p=0.002; GGe0.327). Thus all significant effect can be seen in the 3-way interaction of Condition × Location × Time, of which the mean values and 95% CI are displayed in Figure 2. Confidence intervals reveal that at FP1, FP2, F3, and at some points in time (minute 2 and 4) DC potential of the Mozart condition is more negative than DC potentials of all other conditions. DC potentials of the Mozart condition is also more negative on some other location compared to conditions at some time points. However, the main effect of condition (F(3,111)=2.93; p=0.062; GGe=0.638) failed two-tailed statistical significance of p=0.05.
Figure 2: Mean values and 95% CI of brain DC potential shifts for the four experimental conditions by recording location and time.
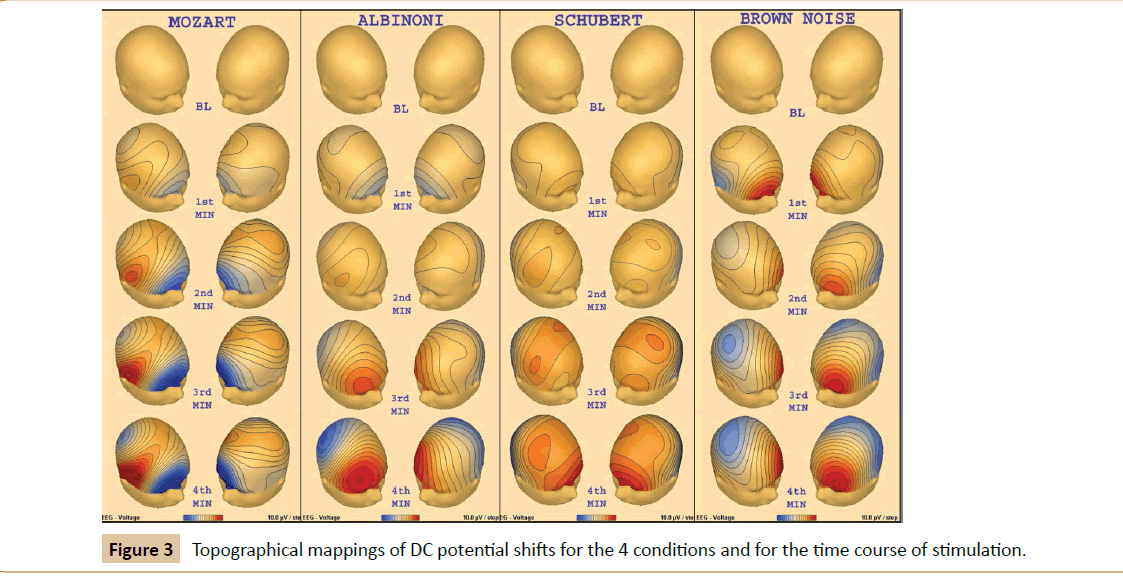
The topographical mappings of DC potential shifts are presented in Figure 3.
Discussion
Results display negative brain DC potential shifts for Mozart’s sonata K. 448 and positive potentials for the two other pieces of music, namely Albinoni’s Adagio in g-minor and Schubert’s Fantasia in f-minor for Piano Duet, D. 940, as well as for brown noise. This indicates a specific activation pattern for the stimulation by Mozart’s sonata only. The pattern of negative shifting DC potentials relative to the baseline is widespread, but most pronounced at prefrontal and frontal sites. A comparable pattern was observed in a foregoing study investigating brain DC potentials under conditions of environmentally vs. internally oriented attention [49] for the environment oriented direction of attention. However, the negative shifted DC potential disappeared under conditions of environmental background noise, which was interpreted as a filter mechanism activated by attention switching. In analogy to the present study this would indicate that Mozart’s sonata induced enhanced attention for processing environmental information, whereas the other pieces of music and also the brown noise stimulation activated an environmental rejection mode of attention. Such an environmental rejection mode associated with positive brain DC potentials was already observed in an animal study, as a response to an averse stimulation [63] and also in humans working on a computer task under environmental background noise conditions contrary to the condition without background noise [64].
The significance of negative brain DC potentials, or at least of negative potential shifts, was already demonstrated for a complex cognitive task of concept formation [43] but also for accuracy and speed in psychomotoric response [65,66] and fit well in the framework of the activation theory as proposed by Lindsley [67] for which specific brain potentials were suggested [42].
In particular the activation of prefrontal (and frontal) cortical areas fits well in foregoing studies by means of other measures like EEG brain waves [36,37], EEG coherence analysis [68], nearinfrared spectroscopy [6], or with fMRI data [40]. There is also a converging view that the activation of networks important for spatial reasoning would be primed by Mozart’s sonata K. 448 [6,37,39,40,69].
If one asks why in particular Mozart’s Sonata K. 448 has the property to activate neuronal cortical circuits related to attentive and cognitive functions, a speculative explanation should be offered. A unique characteristic of hearing Mozart’s Sonata K. 448 is the very specific stimulation by two different, but highly similar musical streams coming from two pianos. One may speculate that the brain is splitting this spatial information in an automatic mode and by thus the unconscious processing primes the processing of spatial information. Consequently, after priming the brain with the Sonata K. 448, a better performance in a subsequent spatial task may be observed.
As this study was well controlled by three control conditions, the specificity of the observed effect seems obvious. Moreover, as there were no significant differences in autonomic activation observed between the four conditions, this indicates that the observed Mozart effect may – at least – be present without an autonomic activation as proposed earlier. Also, mood was not related to the observed effect for the stimulation by Mozart’s sonata, as there was no relevant difference between the three music pieces, but brain DC potentials differed, and mood for brown noise was negative but brain DC potentials were not different to the two other control conditions. Thus, the present study does not support the view that mood [34] or arousal [4,12,54,70] would be the underlying factors of the Mozart effect as also suggested by others [7].
References
- Rauscher FH, Shaw GL, Ky KN (1993) Music and spatial task performance. Nature 365:611.
- Rauscher FH, Shaw GL, Ky KN (1995) Listening to Mozart enhances spatial-temporal reasoning Towards a neurophysiological basis. Neuroscience Letters185:44-7.
- Rideout BE, Taylor J (1997) Enhanced spatial performance following 10 minutes exposure to music: a replication. Perceptual and Motor Skills 85:112-4.
- Thompson WF, Schellenberg EG, Husain G (2001) Arousal, mood, and the Mozart effect. American Psychologist 12:248-251.
- Aoun P, Jones T, Shaw GL, Bodner M (2005) Long-term enhancement of maze learning in mice via a generalized Mozart effect. Neurol Res 27:791-796.
- Suda M, Morimoto K, Obata A, Koizumi H, Maki A. Cortical responses to Mozart's sonata enhance spatial-reasoning ability. Neurological Research. 2008;30(9):885-8.
- Smith A, Waters B, Jones H (2010) Effects of prior exposure to office noise and music on aspects of working memory. Noise Health 12:235-243.
- 8.Borella E, Carretti B, Grassi M, Nucci M, Sciore R. (2014) Are age-related differences between young and older adults in an affective working memory test sensitive to the music effects? Front Aging Neurosci 6:298.
- 9.Xing Y, Chen W, Wang Y, Jing W, GaoS,et al. (2016) Music exposure improves spatial cognition by enhancing the BDNF level of dorsal hippocampal subregions in the developing rats. Brain Res Bull 121:131-137.
- Stough C, Kerkin B, Bates T, Mangan G (1994) Music and spatial IQ .Personality and Individual Differences 17:695.
- Steele KM, Ball TN, Runk R (1997) Listening to Mozart does not enhance backwards digit span performance. Percept Mot Skills 84:1179-1184.
- Steele KM, Brown JD, Stoecker JA (1999) Failure to confirm the Rauscher and Shaw description of recovery of the Mozart effect. Percept Mot Skills 88:843-848.
- Fudin R, Lembessis E (2004) The Mozart effect: questions about the seminal findings of Rauscher, Shaw, and colleagues .Percept Mot Skills.98:389-405.
- Steele KM, dalla Bella S, Peretz I, Dunlop T, Dawe LA, et al. (1999) Prelude or requiem for the 'Mozart effect'? Nature 400:827-828.
- Lin LC, Yang RC (2012) Mozart K.545 is as effective as Mozart K.448 in reducing epileptiform discharges in children with epilepsy: The role of harmonics. Dev Med Child Neurol 54:5.
- Grylls EK, Rudnay J, Kinsky M, Baggott A, Wabnitz C, et al. (2013)The mozart effect in children with epileptic EEGs. Archives of Disease in Childhood: Education and Practice Edition 98:86-87.
- Lin LC, Juan CT, Chang HW, Chiang CT, Wei RC, et al. (2013) Mozart K.448 attenuates spontaneous absence seizure and related high-voltage rhythmic spike discharges in Long Evans rats. Epilepsy Res 104:234-240.
- Lin LC, Lee MW, Wei RC, Mok HK, Yang RC (2014) Mozart K.448 listening decreased seizure recurrence and epileptiform discharges in children with first unprovoked seizures: A randomized controlled study.BMC Complement Altern Med 14:17.
- Sakalauskaite D, Praninskiene R, Samaitiene R. (2016) The impact of music on the bioelectrical activity of the brain in children with idiopathic epilepsy. Epilepsia 57:151-152.
- Coppola G, Toro A, Operto FF, Ferrarioli G, Pisano S, et al. (2015) Mozart's music in children with drug-refractory epileptic encephalopathies. Epilepsy Behav 50:18-22.
- Cacciafesta M, Ettorre E, Amici A, Cicconetti P, Martinelli V, et al. (2010) New frontiers of cognitive rehabilitation in geriatric age: the Mozart Effect (ME). Arch GerontolGeriatr 51: e79-82.
- Johnson JK, Cotman CW, Tasaki CS, Shaw GL. (1998) Enhancement of spatial-temporal reasoning after a Mozart listening condition in Alzheimer's disease: A case study. Neurol Res 20:666-672.
- Wiseman MC, Wiseman DM, Litman L (2011) The augmentation of laparoscopic skill acquisition using the mozarteffect.J Minim Invasive Gynecol 18:68.
- Dugan J, Patel P, Batra S, Wolf D, Kulkarni D ( 2013) Effect of ambient music on adenoma detection rate in endoscopists (emdare) study.Am J Gastroenterol 108:S611-S2.
- Liu B, Huang Y, Wang Z, Wu G (2012) The influence of background music on recognition processes of Chinese characters: an ERP study. NeurosciLett 518:80-85.
- Shue B, Chatterjee A, Fudemberg S, Jay Katz L, Moster MR, et al. (2011) The Effects of Mozart's music on the performance of glaucoma patients on automated perimetry .Invest Ophthalmol Vis Sci 52:7347-7349.
- Bock O (2010) Sensorimotor adaptation is influenced by background music. Exp Brain Res 203:737-741.
- Shaw GL, Silverman DJ, Pearson JC (1985) Model of cortical organization embodying a basis for a theory of information processing and memory recall.ProcNatlAcadSci USA 82:2364-2368.
- Mountcastle VB (1978) An organizing principle for cerebral function. In: Edelman GM, Mountcastle VB, editors. The Mindful Brain. Cambridge: MIT; p.1-50.
- HEBB DO (1955) Drives and the C.N.S. (conceptual nervous system). Psychol Rev 62:243-254.
- Steele KM (2000) Arousal and mood factors in the "Mozart effect". Percept Mot Skills 91:188-190.
- Rideout BE (1999) Performance suppression from control procedures is not the basis of the Mozart effect. Percept Mot Skills 89:890.
- Rideout BE, Dougherty S, Wernert L (1998) Effect of music on spatial performance: a test of generality. Percept Mot Skills 86: 512-514.
- Wang A, Tranel D, Denburg N (2013) The cognitive effects of music: Working memory is enhanced in healthy older adults after listening to music. Neurology 80: Supplement P07.162.
- Rideout BE, Laubach CM (1996) EEG correlates of enhanced spatial performance following exposure to music. Percept Mot Skills 82:427-432.
- Jaušovec N, Habe K (2003) The "Mozart Effect": An Electroencephalographic Analysis Employing the Methods of Induced Event-Related Desynchronization/ Synchronization and Event-Related Coherence. Brain Topography 16:73-84.
- Verrusio W, Ettorre E, Vicenzini E, Vanacore N4, Cacciafesta M, et al. (2015) The Mozart Effect: A quantitative EEG study. Conscious Cogn 35: 150-155.
- Jausovec N, Habe K (2005) The influence of Mozart's sonata K. 448 on brain activity during the performance of spatial rotation and numerical tasks. Brain Topogr 17:207-218.
- Jausovec N, Jausovec K, Gerlic I (2006) The influence of Mozart's music on brain activity in the process of learning. ClinNeurophysiol 117:2703-2714.
- Bodner M, Muftuler LT, Nalcioglu O, Shaw GL (2001) fMRI study relevant to the mozart effect: Brain areas involved in spatial-temporal reasoning. Neurological Research 23:683-690.
- Trimmel M, Groll-Knapp E (1984) On minimization of the electrode bias potential for DC recordings. EEG-Labor 6:13-23.
- Haider M, Groll-Knapp E, Ganglberger JA (1981) Event-related slow (DC) potentials in the human brain. Rev PhysiolBiochemPharmacol 88:125-197.
- Bauer H, Nirnberger G (1979) Paired associate learning with feedback of DC potential shifts of the cerebral cortex. Arch Psychol (Frankf) 132:237-238.
- Trimmel M. Angewandte und ExperimentelleNeuropsychophysiologie. Berlin Heidelberg: Springer-Verlag; 1999.
- Rösler F, Heil M, Röder B (1997) Slow negative brain potentials as reflections of specific modular resources of cognition. BiolPsychol 45:109-141.
- Pirch JH (1980) Correlation Between Steady Potential Baseline and Event-Related Slow Potential Magnitude in the Rat. Int. J. Neurosci 11:25-33.
- Lamm C, Bauer H, Vitouch O, Durec S, Gronister R, et al. (2001) Restriction of task processing time affects cortical activity during processing of a cognitive task: an event-related slow cortical potential study. Cogn Brain Res.10:275-282.
- Bauer H, Birbaumer N, Rösler F. Slow Scalp Recorded Brain Potentials, Sensory Processing and Cognition. In: Laming PR, Syková E, Reichenbach A, Hatton GI, Bauer H, editors. Glial Cells: Their Role in Behavior. Cambridge: Cambridge University Press; 1998. p. 267-90.
- Trimmel K, Schätzer J, Trimmel M (2014) Acoustic noise alters selective attention processes as indicated by direct current (DC) brain potential changes. Int J Environ Res Public Health 11:9938-9953.
- Trimmel M, Wright N, Backs RW (2003) Psychophysiology in Ergonomics: Preface to the Special Section. Human Factors 45:523-524.
- Trimmel M, Fairclough S, Henning R (2009) Psychophysiology in ergonomics. ApplErgon 40: 963-964.
- Petsche H, Pockberger H, Rappelsberger R (1985) Music reception, EEG and musical training. EEG-EMG Zeitschrift fur ElektroenzephalographieElektromyographie und VerwandteGebiete 16:183-190.
- Twomey A, Esgate A (2002) The Mozart effect may only be demonstrable in nonmusicians. Percept Mot Skills 95:1013-1026.
- Nantais KM, Schellenberg EG (1999) The Mozart Effect: An Artifact of Preference. PsycholSci 10:370-373.
- Steyer R. Der mehrdimensionaleBefindlichkeitsfragebogen: (MDBF). Go¨ttingen [u.a.]: Hogrefe, Verl. fu¨rPsychologie; 1997.
- Einthoven W (1903) Die galvanometrischeRegistrirung des menschlichenElektrokardiogramms, zugleicheineBeurteilung der Anwendung des Capillarelektrometers in der Physiologie. Archivfür die gesammtePhysiologie des Menschen und der Thiere (99):472-480.
- Bauer H, Korunka C, Leodolter M (1989) Technical requirements for high-quality scalp DC recordings. ElectromyogrClinNeurophysiol 72:545-7.
- Picton TW, Hillyard SA (1972) Cephalic skin potentials in electroencephalography. ElectromyogrClinNeurophysiol 33:419-424.
- Jasper HH (1958) Report of the committee on methods of clinical examination in electroencephalography. ElectromyogrClinNeurophysiol 10:370-375.
- Stephenson WA, Gibbs FA (1951) A balanced non-cephalic reference electrode. ElectromyogrClinNeurophysiol 3:237-240.
- Pascual-Marqui RD, Gonzalez-Andino SL, Valdes-Sosa PA, Biscay-Lirio R (1988) Current source density estimation and interpolation based on the spherical harmonic Fourier expansion. Int. J. Neurosci 43:237-249.
- Perrin F, Pernier J, Bertrand O (1987) Mapping of scalp potentials by surface spline interpolation. ElectromyogrClinNeurophysiol 66:75-81.
- Haider M, Groll-Knapp E, Trimmel M. Cortical DC-shifts related to sustained sensory stimulation and motor activity. In: Deecke L, Eccles JC, Mountcastle VB, editors. From Neuron to Action. Berlin: Springer; 1990. p. 59-64.
- Trimmel M, Poelzl G (2006) Impact of background noise on reaction time and brain DC potential changes of VDT-based spatial attention. Ergonomics 49: 202-208.
- Born J, Whipple SC, Stamm J (1982) Spontaneous cortical slow-potential shifts and choice reaction time performance. ElectromyogrClinNeurophysiol 54:668-76.
- Trimmel M, Meixner-Pendleton M. Relationships of brain DC potentials to vigilance based ERPs and operator functional state. In: Hockey GRJ, Gaillard AWK, Burov O, editors. Operator functional state: The assessment and prediction of human performance degradation in complex tasks. NATO Science Series I: Life and Behavioral Sciences. 355. Amsterdam: IOS; 2003. p. 131-9.
- Lindsley DB (1961) The reticular activating system and perceptual integration. In: Sheer DE, editor. Electrical stimulation of the brain. Austin, TX: University of Texas Press. p. 331-49.
- Sarnthein J, VonStein A, Rappelsberger P, Petsche H, Rauscher FH, et.al. (1997) Persistent patterns of brain activity: An EEG coherence study of the positive effect of music on spatial-temporal reasoning. Neurol Res 19:107-116.
- Rauscher FH, Shaw GL, Levine LJ, Wright EL, Dennis WR, et.al. (1997) Music training causes long-term enhancement of preschool children's spatial-temporal reasoning. Neurol Res 19:2-8.
- Husain G, Thompson WF, Schellenberg EG (2002) Effects of Musical Tempo and Mode on Arousal, Mood, and Spatial Abilities. Music Perception: Interdiscip J 20:151-171.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences