Bio Fertilizer from Egyptian Bahraiya Oasis and Aswan Iron Ore Impurities Bioleaching by Azotobacter vinelandii
Mohamed Ali El-Badry Hafez1*, Elbarbary TA2, Ibrahim IA2 and Abdel-Fatah YM2
1Department of Botany and Microbiology, Al-Azher University, Cairo, Egypt
2Central Metallurgical Research and Development Institute, Helwan, Cairo, Egypt
- *Corresponding Author:
- Mohamed Ali El-Badry Hafez
Department of Botany and Microbiology
Faculty of Science, Al-Azher University
Cairo, Egypt
Tel: +201005077625
E-mail: mohamed.helwanuni@yahoo.com
Received Date: 22 September 2017; Accepted Date: 12 October 2017; Published Date: 19 October 2017
Citation: El-Badry Hafez MA, Elbarbary TA, Ibrahim IA, Abdel-Fatah YM (2017) Bio Fertilizer from Egyptian Bahraiya Oasis and Aswan Iron Ore Impurities Bioleaching by Azotobacter vinelandii. J Mol Microbiol. Vol. 1 No. 1: 4.
Copyright: © 2017 El-Badry Hafez MA, et al. This is an open-access article distributed under the terms of the creative Commons attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In order to overcome of expensive and extensive use of chemical phosphate fertilizers, in this work we investigated the potential of phosphate dissolution by sustainable industries impurities recovery using Azotobacter vinelandii, dissolution of phosphates impurities from two Egyptian iron ores Bahraiya Oasis and Aswan, A. vinelandii produces low molecular weight organic acids, which attack the phosphate structure in iron ores and convert phosphorus from non-utilizable form to the plants. The objective of the present work was to bioleach of phosphate impurities and study the factors influence on dissolution of phosphate content in iron ores Bahraiya oasis and Aswan, Egypt. The optimum factors increase bioleaching of phosphate impurities from two Egyptian iron ores were three days incubation time, cultivated in modified PVK medium for dissolution of phosphate from two iron ore, 0.1 × 1029 colony forming unit of A. vinelandii, 0.5 g iron ore concentration, at incubation temperature 30°C, ammonium oxalate as nitrogen source, glucose as carbon source, no significant effect of addition factor, also there is decreasing in pH and increases in electric potential, initial pH 7, which the leaching efficiency of phosphate content in iron ore reaches to 73.4% and 60.53% for Bhraiya Oasis and Aswan and respectively.
Keywords
Iron ore; Fertilizers; Bioleaching; Azotobacter vinelandii
Introduction
Phosphate, mostly found as an ingredient of iron ore, which neglected in the manufacture of iron and steel. Heat handling and following by leaching was the best a way for rise high phosphate obtained from iron ores [1]. Low prices in the recent past for this commodity frustrated industrial adoption of hydrometallurgical beneficiation of such ores. Nowadays, an increase in universal steel production had been increased demand for iron ore with a consequent growing in the price for this ware, making hydrometallurgical of phosphate impurities removal viable from iron ore [2]. Bio hydrometallurgy considered as way for the elimination of phosphate impurities from iron ores because it is well settled that microorganisms, especially in nutrient-limited environments, are capable of mobilizing the phosphorus contained in minerals [3]. Some studies have been published on phosphate removal from iron ores using microorganisms, including filamentous fungi and iron-oxidizing bacteria [4]. Heterotrophic phosphate bioleaching from an iron slag using the bacterium Frateuria aurantia has been reported by Pradhan et al. [5]. The use of microorganisms indigenous to an iron ore can have some merits in bio beneficiation in terms of either incubation time of the microorganisms to a bio beneficiation process or in ecological disruptions in the surrounding area [6].
In the present study, the possibility of removing phosphate impurities from Egyptian iron ores (Bahraiya Oasis and Aswan which contain 0.45 and 0.81% respectively using a A. vinelandii and evaluate the factors effect on dissolution of phosphate content from iron ore to maximize dissolution percentage of P2O5 from two iron ore.
Materials and Methods
Ore
Bahraiya Oasis and Aswan iron ore sample is collected in plastic bags from Iron and Steel Company, Aswan iron Ore collected from Aswan by special communications. Chemical composition of Iron ore Aswan and Bahraiya Oasis were determined by using XRD and XRF analysis.
Microorganism
Azotobacter vinelandii was previously isolated, identified and evaluated previously by El-Badry et al. [7], for its ability for phosphate ore dissolution to produces phosphorus fertilizers.
Culture media
Modified Pikovskaya’s medium: This media contains (g/l): 0.5 Yeast extract, 10 Dextrose, 0.5 Ammonium sulphate, 0.2 Potassium chloride, 0.1 Magnesium sulphate, 0.0001 Manganese sulphate and 0.0001 Ferrous sulphate. Suspend 16.3 grams in 1000 ml distilled water, Sterilization by autoclaving at 15 lbs pressure and (121°C) for 15 minutes dispense as desired. This medium is solidified by adding 15 g agar per liter [8].
Experiment method: Prepare 50 ml of modified PVK broth medium in 100 ml conical flask with 0.25 g sterilized iron ore inoculated by A. vinelandii and control without bacteria then incubated it in shaking incubator at 30°C and 160 rpm. A 5mL cell free filtrate was removed from each flask after 0, 1, 2, 3, 4 and 5 days of reaction then centrifuged at 9000 rpm for 10 minutes. The amount of soluble phosphate of iron ore filtrate was determined by calorimetrically methods according Olsen et al. [9].
Effect of different growth parameter on phosphate solublization: A. vinelandii was grown in 100 ml Erlenmeyer flasks containing 50 ml of different growth culture media PVK medium, modified PVK medium, Ashyb’s medium and nutrient agar medium separately then supplemented with 0.25 g of Bahraiya Oasis and Aswan iron ore for 50 ml medium. Each flask is inoculated with 0.1 × 1029 cfu of A. vinelandii isolates and incubated at 30°C. The amount of soluble phosphate in the culture filtrate was determined. The previous steps are conducted on modified pikovskaya’s medium supplemented with Bahraiya Oasis and Aswan iron ore for A. vinelandii at different incubation periods, temperatures, ore weight, bacterial inoculum size, different carbon, and nitrogen sources and initial pH.
Results and Discussion
Chemical composition of Iron ore bahraiya oasis
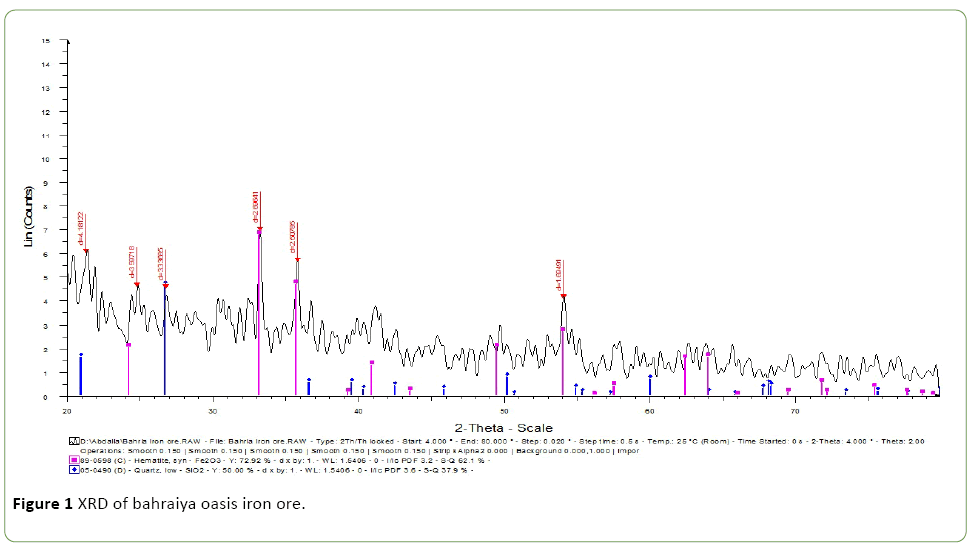
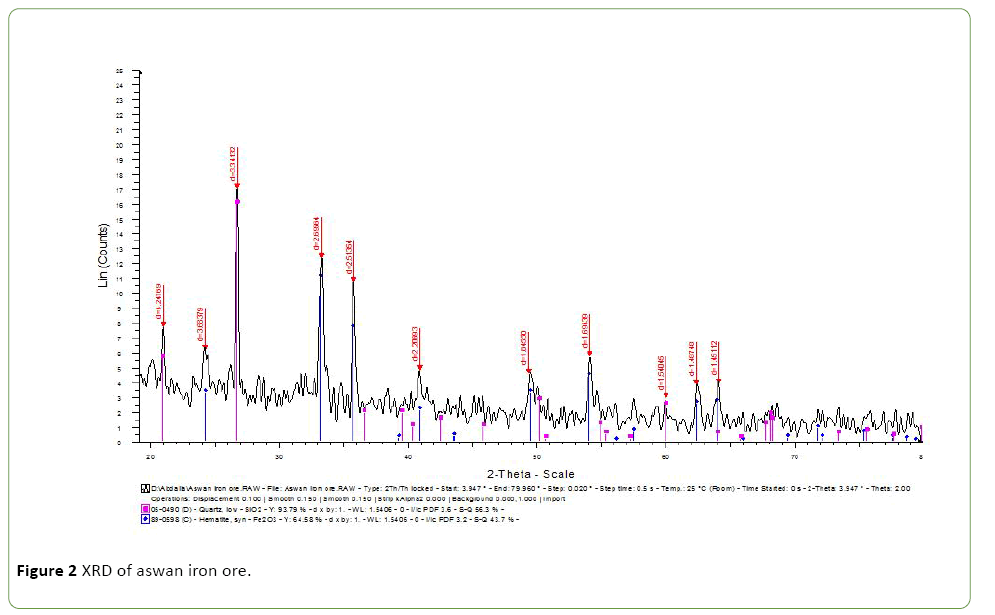
Bahraiya Oasis and Aswan iron ore characterized by XRD Figures 1 and 2 which show the presence of Iron and silica mineral in both figures the chemical analysis by XRF are tabulated in Tables 1 and 2.
| Component | % | Component | % |
|---|---|---|---|
| Na2O | 0.38 | MnO | 6 |
| MgO | 0.35 | Fe2O3 | 70.57 |
| Al2O3 | 2.25 | BaO | 0.85 |
| SiO2 | 7.15 | ZnO | 0.1 |
| P2O5 | 0.45 | Cl | 0.48 |
| S | 0.58 | K2O | 0.39 |
| CaO | 0.12 |
Table 1 XRF of El-bahraiya oasis iron ore.
| Component | % | Component | % |
|---|---|---|---|
| Na2O | 0.157 | MnO | 0.376 |
| MgO | 0.491 | Fe2O3 | 72.903 |
| Al2O3 | 4..030 | NiO | 0.075 |
| SiO2 | 13.466 | CuO | 0.041 |
| P2O5 | 0.808 | ZnO | 0.029 |
| SO3 | 0.771 | Rb2O | 0.005 |
| K2O | 0.185 | SrO | 0.116 |
| CaO | 1.803 | Y2O3 | 0.016 |
| TiO2 | 0.453 | ZrO2 | 0.059 |
| V2O5 | 0.077 | BaO | 0.82 |
| Cr2O3 | 0.026 | CeO2 | 0.036 |
| L.O.I. | 4 | Br | 0.021 |
Table 2 XRF of aswan iron ore.
The presence percentage of P2O5 as impurities in these two iron ore were evaluated to bioleaching them by A. vinelandii as El-Bahraiya Oasis Iron Ore P2O5 was 0.45% whereas Aswan Iron Ore was higher P2O5 impurities with 0.808.
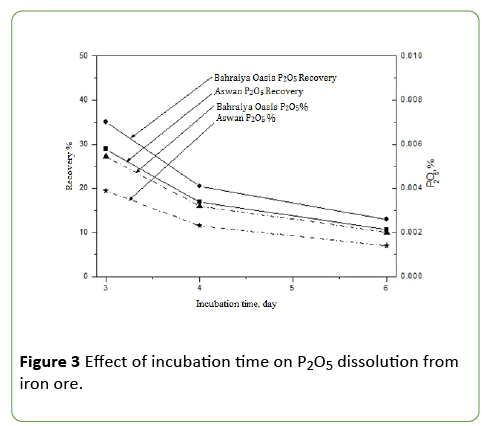
Evaluation of incubation period on phosphate impurities dissolution from iron ores by A. vinelandii
PVK medium in presence 0.25 g Bahraiya Oasis ore and Aswan Iron ores for 50 ml of medium and inoculated with 0.5 × 1029 cfu of A. vinelandii and measuring P2O5 daily. The results revealed that maximum phosphate dissolution was obtained after three day which reaches to 35% and 28% P2O5 recovery for Bahraiya Oasis ore and Aswan respectively. All data showed in Figure 3. Dissolution of rock phosphate depends on its structural complexity, particle size and metabolites of microorganism [10].
This work agrees with Rahim et al. [11]. That show A. vinelandii delayed for 72 hour and reached at stationary phase and also noticeable reduction in phosphate solubilizing index during stationary phase of growth found in all cases supports the dependence of phosphate solubilizing index on bacterial metabolism. It was also shown that the phosphatase activity of bacterial strain could synergistically enhance the release of Pi in the acidified medium. The merits of bacteria capable of phosphate dissolution with simultaneous production of organic acids and phosphatase enzyme activity on production and yield were shown in both green house and field trials [12].
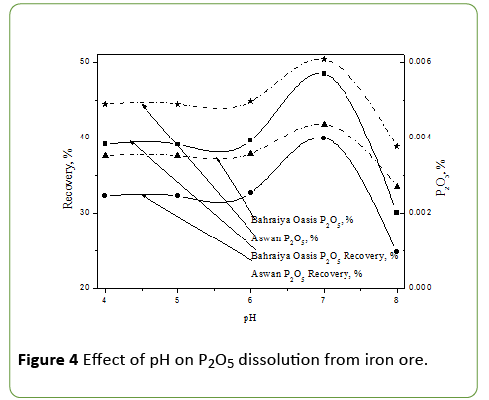
Evaluation of different initial pH on phosphate impurities dissolution from iron ores by A. vinelandii
Different initial pH (4, 5, 6, 7 and 8) of 50 ml of modified PVK medium in presence 0.25 g Bahraiya Oasis and also for Aswan iron ore and inoculated with 0.5 × 1029 cfu of A. vinelandii in 100 ml flask and incubated at 30°C and 160 rpm and measuring P2O5 after 3 day of incubation was determined.
Phosphate dissolution was affected with different initial pH of the modified PVK medium as in the Figure 4.
The maximum growth of bacterium on a medium containing iron ore is observed at initial pH 7. At this pH value phosphate solublization exhibited high amounts it represented 48.4% and 39.9% recovery of P2O5, and 0.00607% and 0.00436 for P2O5 dissolution for Bahraiya oasis and Aswan Iron ore. It is also observed that phosphate solublization at pH 5 was sharply decreased and this agree with Tejera et al. [13]. Showed that a lower number of isolates grew on N-free media at pH value as high as 8.7, the above results were agreed with El-Badry et al. [7].
The pH of the culture medium affect the growth behavior of microorganisms and the biochemical reactions which they perform, in many cases, acidification is the main mechanism involved in phosphate dissolution [14]. However, several studies have shown a lack of correlation between solubilized phosphorus and pH of the medium [15].
Several authors have suggested that a decrease in pH due to the production of organic acids and the release of protons is a basic principle of phosphate dissolution [16]. There are several dissolution mechanisms were involved at the pH of the medium varies. These mechanisms can be: proton exclusion (via cellular respiration and ammonium absorption as N source) [17]. Siderophores [18] and exopolisaccharide (EPS) production, the production of EPS that could act synergistically with acid production as suggested by Yi et al. [19].
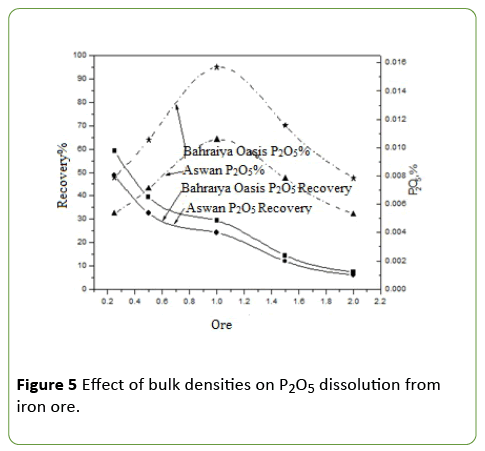
Evaluation of different ore weight on phosphate impurities dissolution from iron ores by A. vinelandii
It was evaluated by using four different weights of two iron ore separately as (0.25, 0.5, 1, 2) g with above optimum conditions.
A. vinelandii isolate has varied growth in the presence of different concentrations of phosphate ore in the growth medium up to 4% (Figure 5). The optimum growth and best phosphate dissolution occurred at a concentration of 0.5% of the iron ore concentration and decreased above this concentration. It is also observed decrease pH value and no change highly between various concentrations of ores; this may be due to the production of organic acids and acidic phosphatase enzymes. At a concentration of 0.5% ore, A. vinelandii isolate can solubilize approximately 59.2%, 48.8% P2O5 recovery and, 0.01568, 0.01057 for P2O5 dissolution percentage of Bahraiya Oasis and Aswan Iron ore respectively.
The dissolution of phosphate decreases with increasing iron ore concentration in the growth medium, that may be attributed to toxic effect of some metal ions which may be released into the culture medium such as Fe3+, Mn+2, Na+1 and, Ca+2 ions and these ions can react with soluble phosphate and form insoluble phosphate so decrease total soluble phosphate, these results found to be almost similar to that obtained by Hefnawy et al. Also, it may be due to inhibitory effect on further phosphate solubilization [20]. The negative effect of dissolve phosphate on microbial acid productivity [21], Might also be responsible for final soluble phosphate concentration. Another explanation for this might be formation of an organo-P compound induced by organic metabolites released, which in turn, reduces the amount of available P [17].
The adverse effect of increasing pulp density could be attributed to the inhibitory effect of increasing concentrations of ferric iron, the limited availability of nutrients and, O2 and CO2 with increasing pulp density and the mechanical damage to bacterial cells by solids [22].
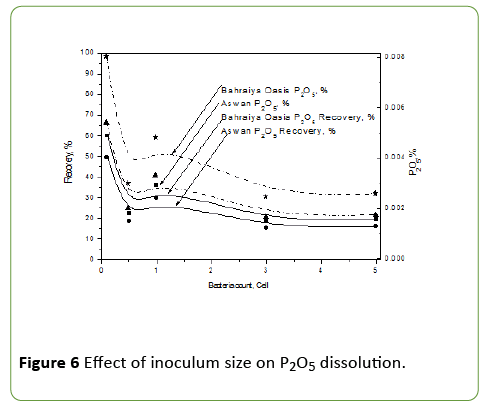
Evaluation of different inoculum size on phosphate impurities dissolution from iron ores by A. vinelandii
It was studied by using four different inoculum size (0.1 × 1029, 0.5 ×1029, 1 ×1029, 3 × 1029, 5 × 1029) cfu and above optimum conditions was applied.
The best phosphate dissolution occurred at a concentration of 0.1 × 1029 cfu of A. vinelandii and decrease at high concentration of it with no highly change in final pH value and this may be due to competition between bacterial cells themselves, decrease the aeration and also high growth which may consume phosphate. At a concentration of 0.1 × 1029 cfu of A. vinelandii solubilized approximately 60, 49.4 P2O5 recovery percentage and 0.00802, 0.0054 P2O5 dissolution percentage for Bahraiya Oasis and Aswan iron ore respectively. All data are plotted in Figure 6.
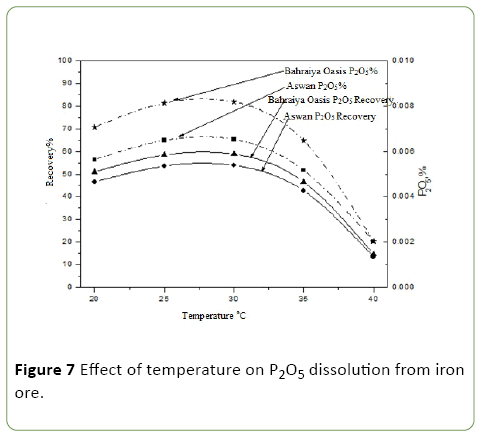
Evaluation of different incubation temperatures on phosphate impurities dissolution from iron ores by A. vinelandii
It was studied by using four different temperatures (20, 30, 40, 50°C) and above optimum conditions was applied (Figure 7).
The dissolution of phosphate content of ore increase with increase the temperature of incubation up to 30°C then begins decrease and dissolution of phosphate content of ore reach to 65.3, 53.8 P2O5 recovery percentage and 0.00819, 0.00588 P2O5 dissolution percentage for Bahraiya Oasis and Aswan iron ore so the optimum incubation temperature for best phosphate solublization activity by A. vinelandii isolate is 30°C at which optimum growth and adapt to their indigenous environment so their metabolic activities are linked to the temperature of the environment [23-25].
This agrees with Rahim et al. [11] which bacterial growth and consequently PSI of bacteria were reduced at both high and low temperatures and also agrees with Neeru et al. [26]. The growth of Bacterium at 30°C refers to mesophilic bacterium which grows best in moderate temperature, neither too hot nor too cold [27].
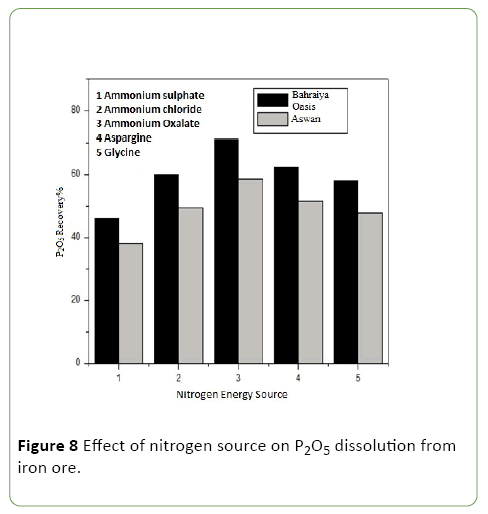
Evaluation of different nitrogen sources on phosphate impurities dissolution from iron ores by A. vinelandii
It was evaluated by using five different nitrogen sources of (ammonium sulphate, ammonium chloride, ammonium oxalate, asparagine, and glycine) and above optimum conditions was applied (Figure 8).
A. vinelandii dissolve high amount of phosphorus from iron ore with all tested nitrogen sources, Ammonium oxalate was found to be the best nitrogen source for maximum phosphate dissolution which reaches to 50.8% followed by asparagine and lowest dissolution of phosphate content of the ore at using ammonium chloride as nitrogen source.
As a nitrogen source, ammonium oxalate was found to give maximum soluble P2O5 which reach to 71.03, 58.57 P2O5 recovery percentage and 0.0089, 0.0064. P2O5 percentage for Bahraiya oasis and Aswan iron respectively –Oxalate ions have the ability to form stable complexes with calcium, iron and aluminum to liberate phosphates [28], and are known to extract P from soils [29,30]. Phosphate dissolution was correlated with the assimilation of both ammonium and chelation by oxalate ions in the culture medium and this observation may be attributed to the release of protons from the cytoplasm to the outer surface leading to dissolution of phosphate content of ore [31].
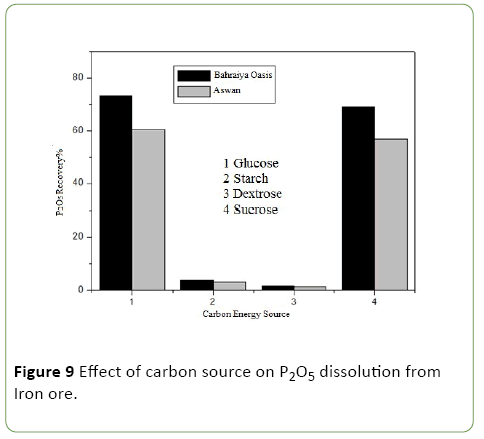
Evaluation of different carbon sources on phosphate impurities dissolution from iron ores by A. vinelandii
It was evaluated by using four different carbon source of (glucose, starch, dextrose, sucrose) and above optimum conditions was applied. (Figure 9).
The results revealed that A. vinelandii grows well on modified PVK liquid medium containing different carbon sources. Whereas, high amounts of soluble phosphate was detected only in the culture filtrate with glucose which reaches to 52.8% then dextrose with low pH value, while starch and sucrose exhibited low amount of soluble phosphate with high pH value. The bacterial growth exhibited remarkable variation according to the utilized carbon source, the best bacterial growth to produce enzyme and organic acids reached when glucose is utilized as a carbon source. The maximum amount of phosphorus solubilized corresponded to the highest value of the organic acid produced which reach to 73.4, 60.53 for P2O5 recovery percentage and 0.00981, 0.0066 for P2O5 dissolution percentage. The sugar consumption and organic acid liberation were seen to be most active up to 3 day. It is generally accepted that the release of insoluble and fixed forms of P carried out by the action of phosphate-solubilizing bacteria via the secretion of low molecular weight organic acids mainly gluconic and keto-gluconic acids and phosphatases. These acids are produced in the periplasm of many Gram-negative bacteria through a direct oxidation pathway of glucose (DOPG, non-phosphorylating oxidation), consequently, the organic acids diffuse freely outside the cells and may release high amounts of soluble P from mineral phosphates, by supplying both protons and metal complex organic acid anions [32].
Also this result agrees with Sridevi et al. [33]. That how glucose was the best carbon source for phosphate solubilization. The maximum decrease in pH was recorded in glucose-containing medium. In other carbon sources little decrease in pH and no correlation between acidic pH and quantity of P2O5 liberated were observed. And also this result reported earlier in Bradyrhizobium species isolated from Cicer arietinum [34].
Conclusion
The presence of phosphate in iron ore reduce its value and complicate its industrial processes. So, in this work, the ability of Azotobacter vinelandii in dissolution of phosphate ores was applied to eliminate the phosphate impurities from two Egyptian iron ores Baharhia osis and Aswan. Phosphate produced as P2O5 which can be used as bio fertilizer.
References
- Araujo A, Walker JW (1994) Kinetics of tension development in skinned cardiac myocytes measured by photorelease of Ca2+. Am J Physiol 267: 1643-1653.
- Kokal HR, Singh MP, Naydyonov VA (2003) Removal of phosphorus from Lisakovsky iron ore by roast-leach process. Wiley Online Library.
- Banfield JF, Barker WW, Welch SA, Taunton A (1999) Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. ProcNatl Acad Sci U S A 96: 3404-3411.
- Parks EJ, Olson GJ, Brinckman FE, Baldi F (1990) Characterization by high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by a fungus. J Ind Microbiol Biotechnol 5: 183-218.
- Pradhan N, Das B, Acharya S, Kar RN, Sukla LB, et al. (2004) Removal of phosphorus from LD slag using a heterotrophic bacteria. Miner Metall. Process 21: 149-152.
- Delvasto P, Valverde A, Ballester A, Igual JM, Munoz JA, et al. (2006) Characterization of brushite as a re-crystallization product formed during bacterial solubilization of hydroxyapatite in batch cultures. Soil Biol Biochem 38: 2645-2654.
- El-Badry MA, Elbarbary TA, Ibrahim IA, Abdel-Fatah YM (2016) Azotobactervinelandii Evaluation and Optimization of Abu Tartur Egyptian phosphate ore dissolution. Saudi J Pathol Microbiol 1: 80-93.
- Sundara Rao WVB, Sinha MK (1963) Phosphate dissolving micro-organisms in the soil and rhizosphere. Indian J Agric Sci 33:272–278.
- Olsen SR, Cole CV, Watanabe FS, Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. U S Dep of Agric Circ 939: 18-19.
- Pradhan N, Shukla LB (2005) Solubilization of inorganic phosphates by fungi isolated from agriculture soil. Afr J Biotechnol 5: 850-854.
- Rahim N,Parviz O , Horieh S ,Iraj R, Mohammad A(2014) Phosphate solubilization characteristics of efficient nitrogen fixing soil Azotobacter strains. Iran J Microbiol 6: 285-295.
- Malboobi MA, Behbahani M, Madani H, Owlia P, Deljou A, et al. (2009) Performance evaluation of potent phosphate solubilizing bacteria in potato rhizosphere. World J Microbiol Biotechnol 25: 1479–1484.
- Tejera N, Lluch C, Martinez-Toledo MV, Gonzalez-Lopez J (2005) Isolation and characterization of Azotobacter and Azospirillum strains from the sugarcane rhizosphere. Plant Soil 270: 223–232.
- Marra LM, Sousa Soares CRF, Oliveira SMD, Ferreira PAA, Soares BL, et al. (2012) Biological nitrogen fixation and phosphate solubilization by bacteria isolated from tropical soils. Plant Soil 357: 289-307.
- Chaiharn M, Lumyong S (2009) Phosphate solubilization potential and stress tolerance of rhizobacteria from rice soil in Northern Thailand. World J Microbiol Biotechnol 25: 305-314.
- Sperber JI (1958) The incidence of apatite solubilizing organisms in the rhizosphere and soil. Aust J Agric Res 9: 778-781.
- Illmer P, Schineer F (1992) Solubilization of insoluble phosphates by micro- organisms isolated from forest soils. Soil Biol Biochem 24: 389-395.
- Hamdali H, Bouizgarne B, Hafidi M (2008) Screening for rock phosphate solubilizing Actinomycetes from Moroccan phosphate mines. Appl Soil Ecol 38: 12-19.
- Yi Y, Huang W, Ge Y (2008) Exopolysaccharide: A novel important factor in the microbial dissolution of tricalcium phosphate. World J Microbiol Biotechnol 24: 1059-1065.
- Narsian, V, Thakkar J, Patel HH (1995) Mineral phosphate solubilization by A. aculeatus. Ind J Exp Biol 33: 91-93.
- Rohr M, Kubicek CP, Kominek J (1983) Citric acid. In: Reed G, Rehm HJ (eds.), Biotechnology, Volume 3.Verlag-Chemie, Weinheim.pp: 419-454.
- Venkateshwarlu B, Rao AV, Raina P (1984) Evaluation of phosphorus solubilization by microorganisms isolated from arid soils. J Ind Soc Soil Sci 32: 273-277.
- Shahab S, Ahmed N (2008) Effect of various parameters on the efficiency of zinc phosphate solubilization by indigenous bacterial isolates. Afr J Biotechnol 7: 1543-1549.
- Varsha NHH (2002) Aspergiliusaculeatus as a rock phosphatesolubilizer. Soil Biol Biochem 32:599-565.
- Wakelin SA, Warren RA, Harvey R, Ryder MH (2004) Phosphate solubilization by Penicillium spp. closely associated with wheat roots. Biol Fertil Soils 40: 36-43.
- Narula N, Kukreja K, Kumar V, Lakshminaryana K (2002) Phosphate solubilization by soil isolates of Azotobacterchroococcum and their survival at different temperatures. J Agr Trop Subtrop 103: 81-87.
- Willey, Joanne M, Sherwood L, Christopher J, Woolverton, et al. (2008) Prescott, Harley and Klein's Microbiology. McGraw-Hill Higher Education, New York.
- Saghir MK, Zaidi A, Wani PA (2009) Role of phosphate solubilizing microorganisms in sustainable agriculture.Sust Agric 5: 551-570.
- Narteh LT, Sahrawat KL (1999) A Influence of flooding on electrochemical and chemical properties of West African soils. Geoderma 87: 179-207.
- Narteh LT, Sahrawat KL (1999) Oxalate and EDTA extractable soil phosphorus and iron in relation to P availability in lowland rice soils of West Africa Ghana. J Agric Sci 32: 189-198.
- Illmer P, Schinmer F (1995) Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biol Biochem 27: 257-263.
- Sashidhar B, Podile AR (2010) Review mineral phosphate solubilization by rhizosphere bacteria and scope for manipulation of the direct oxidation pathway involving glucose dehydrogenase. J Appl Microbiol 109: 1-12.
- Sridevi M, Mallaiah K V (2009) Phosphate solubilization by Rhizobium strains. Indian J Microbiol 49:98–101.
- Haldar AK, Mishra AK, Bhattacharya P, Chakrabarty PK (1991) Solubilization of inorganic phosphates by Bradyrhizobium. Indian J Exp Biol 29: 28-31.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences