ISSN : 2347-5447
British Biomedical Bulletin
Aspirin and Venous Thrombosis
Karsten Schrör1* and Bernhard H Rauch2
1Institut für Pharmakologie und Klinische Pharmakologie, Heinrich-Heine-Universität Düsseldorf, Moorenstr. 5, Düsseldorf, Germany
2Institut für Pharmakologie, Ernst-Moritz-Arndt-Universität, Felix-Hausdorff-Str. 3, Greifswald, Germany
- *Corresponding Author:
- Schrör K
Institut für Pharmakologie und Klinische Pharmakologie
Heinrich-Heine-Universität Düsseldorf, Moorenstr. 5
40225 Düsseldorf, Germany
Tel: +49 211 81112500
E-mail: schroer.frechen@uni-duesseldorf.de
Received date: January 21, 2017; Accepted date: January 25, 2017; Published date: January 28, 2017
Citation: Schrör K, Rauch BH (2017) Aspirin and Venous Thrombosis. Br Biomed Bull 5:297.
Copyright: © 2017 Schrör K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pharmacological prevention of venous thromboembolism (VTE) is primarily focussed on thrombin inhibitors, mainly, cumarins (warfarin), low molecular weight heparins (LMWH) and, more recently, new, direct acting oral anticoagulants (NOAC). However, there may also be a role for aspirin. Several large, though mostly nonrandomized trials in patients undergoing joint surgery (hip, knee) do suggest that aspirin as part of a multimodal approach is not inferior to warfarin or LMWH in primary prevention of VET. Similar considerations appear to apply for secondary longterm prevention of recurrent VTE after the end of guide-line directed anticoagulation. New experimental data in mouse models of VTE demonstrate a potent, partially thromboxane-mediated, antithrombotic action of low-dose aspirin which is related to its antiplatelet effect. In addition, low-dose aspirin and salicylate were also shown to inhibit the local expression of inflammatory genes, including cytokines and COX-2, possible amplifiers of the thrombotic process. These data provide new mechanism-based support for possible benefical actions of aspirin in prevention of VTE which are not shared with warfarin-type anticoagulants or Factor Xa-inhibitor-type NOACs. A final assessment of the role of aspirin in prevention of VTE is not possible yet. Direct head-to-head comparisons with NOAC are indispensable and are in progress (EPCAT-II; EINSTEINCHOICE). The patient´s individual risk profile needs also to be considered. This includes comorbidities, as well as the possible requirement of long term antiplatelet treatment wirth aspirin for prevention of arterial thromboembolism (myocardial infarction).
Keywords
Aspirin; Venous thrombosis; NOAC; Heparins; Warfarin; Clinical studies; Pharmacology
Introduction
Deep vein thrombosis of the limbs (DVT) clinically presenting as venous thromboembolism (VTE) with pulmonary embolism (PE) as the major complication is a dangerous and potentially fatal disease. Rather non-predictable is spontaneous, idiopathic VTE, due to genetic disposition and/or after (guideline-conform) stop of secondary thrombosis prophylaxis after VTE. In contrast, postsurgical VTE is due to an iatrogenic activation of the clotting system by the operative procedure while tumor-associated VET is mainly due to the tumor (metastatic) process itself. Thus, the pathophysiology of the disease is multifactorial and the medical treatment could be as well.
Pharmacologically, inhibition of thrombin formation is the primary target of VTE prevention. Standard drugs are low molecular weight heparins (LMWH) and pentasaccharide (fondaparinux) as well as cumarin-type oral anticoagulants (warfarin) [1]. More recently, direct acting oral anticoagulants (NOAC), i.e. inhibitors of thrombin (dabigatran) [2] or factor Xa (e.g. rivaroxaban, apixaban, edoxaban) have been introduced [3]. However, they also increased bleeding, although this risk might not be the same with all agents [4-6]. They have already largely displaced warfarin-type anticoagulants for stroke prevention in patients with atrial fibrillation because of an improved benefit/risk profile. Beneficial effects were also obtained in long-term prevention trials of recurrent VTE as compared with warfarin-type anticoagulants [7,8]. As had to be expected, they have also been proven to be effective versus placebo in long-term prevention of recurrent venous thromboembolism.
Nevertheless, there might also be a role for antiplatelet agents, such aspirin, in prevention of VTE as part of a multimodal approach, both as extended postsurgical treatment after an initial treatment phase with anticoagulants [9] as well as extended treatment in secondary prevention of unprovoked VTE. Two prospective randomized, placebo-controlled trials have recently shown that low-dose aspirin will have a beneficial effect in prevention of VTE [10]. Though the efficacy was less than that of NOACs or warfarin [11,12], there was also little risk for severe aspirin-induced bleedings which is a relevant factor for longterm or even life-long anticoagulation. In addition, patients with idiopathic, unprovoked VTE might also be at enhanced risk for arterial thromboembolism (myocardial infarction) [13]. Conversely, atherosclerosis is also a risk factor not only for arterial but also for spontaneous VTE [14]. These findings have reanimated the discussion on use of aspirin also for prevention of VTE, specifically the long-term prevention of recurrent VTE after completion of an initial treatment period with anticoagulants. In addition, there are also exciting new data from experimental pharmacology with aspirin, demonstrating highly significant aspirin-sensitive interactions between platelets and (other) inflammatory cells and their mediators in VTE which might also contribute to an improved clinical outcome. Actual reviews on this issue are available [15,16]. This paper discusses the actual status on the mode of antithrombotic action of aspirin in experimental VTE, providing evidence for beneficial actions also in the clinics. This is followed by an overview of clinical studies comparing aspirin and oral anticoagulants in prevention of VTE, focusing also on the new direct acting oral anticoagulants (NOACs) and providing finally an outlook on possible future developments.
Results and Discussion
Aspirin and platelets– new aspects on mode of action in prevention of VTE in experimental settings
The Virchow-triad of venous thrombogenesis and thromboembolism (VTE) does not specify platelets as potential clotting factors but only mentions “blood constituents”. However, functional interactions between (proinflammatory) platelet functions, local inflammatory conditions (varicosis with local stasis) and disturbed endothelial function are well established meanwhile. The clotting process in veins is initiated by the availability of “tissue factor” (TF), subsequent thrombin generation, activation of white cells and platelets and fibrin formation. This occurs at the morphologically intact endothelium – in contrast to plaque rupture-driven TF formation in arterial thromboembolism [17,18]. The developing adherent venous thrombus will grow in direction to the lumen with a luminal increasing proportion of platelets [19]. Inside the clot, thrombin formation becomes the key event for maintaining activation of the clotting system, stimulation of clot-related platelet activity and white cell functions and formation of fibrinrich platelet-white cell aggregates. Exposed phospholipids at the platelet surface strongly propagate the coagulation process by facilitating the assembly and activation of tenase and prothrombinase complexes [20]. Aspirin can inhibit thrombin formation at antiplatelet doses via inhibition of platelet function and/or indirectly, via interaction with clotting factors [21-23]. These data suggest a significant role for platelets and, consequently antiplatelet drugs–on thrombin formation, the driving force for DVT.
In a mouse model of VTE, bearing a thrombin-driven thrombotic phenotype without additional triggers by genetic manipulation, antibody-mediated depletion of platelets fully abrogated the clinical features of VTE, whereas antibodymediated depletion of circulating neutrophils, which were abundant in the thrombotic lesions, did not affect onset, severity, or thrombus morphology [24]. These data confirm platelets as important determinants also for VTE even in an entirely thrombin-driven VTE model. The key role of platelets as well as the efficacy of antiplatelet treatment were confirmed with another thrombin-driven though less severe mouse model of experimental VTE in mice, i.e. subtotal ligation of the inferior caval vein. In this model thrombus formation after exogenous administration of TF was not modified by leukopenia but markedly reduced after thrombocytopenia induced by an antiplatelet antiserum as well as by treatment with an antiplatelet drug (clopidogrel). This effect of clopidogel was not maintained in the presence of an about 80fold higher dose of added TF. However, this was the first paper to demonstrate that antiplatelet agents might inhibit venous thrombus formation by interaction with the plasmatic clotting system [25].
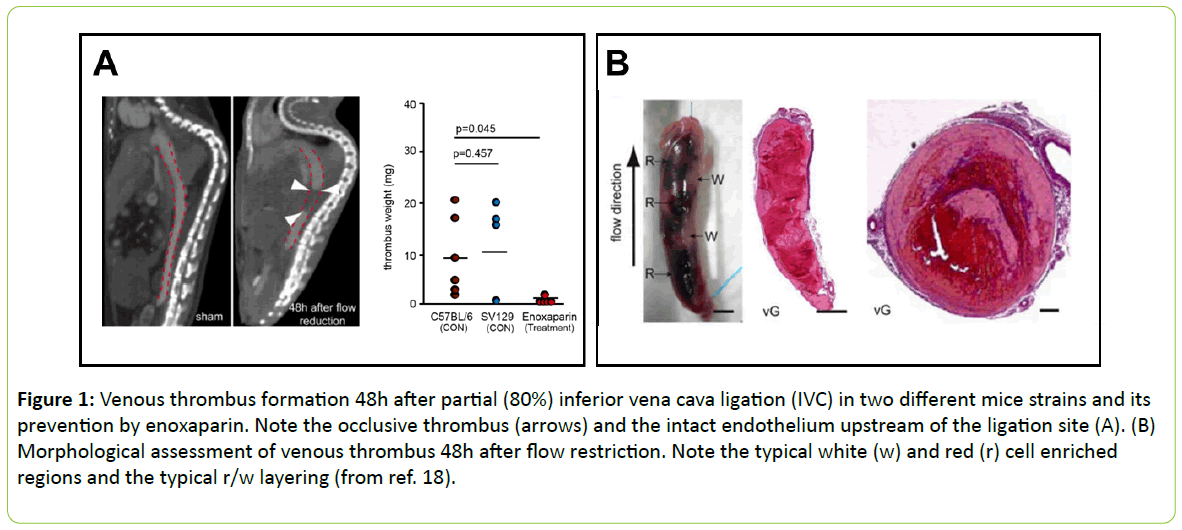
More detailed information about the role of platelets in venous thrombus formation was obtained in a less aggressive variation of this method of inferior caval vein occlusion without addition of external TF. Partial ligation resulted in significant formation of platelet-white cell thrombi within 48h. This model was considered to more closely resemble the time course and clinical features of DVT in man. In this model, blood monocytes and neutrophils crawling along and adhering to the venous endothelium provided the initiating stimulus for DVT development. TF derived from myeloid leukocytes caused extensive intraluminal fibrin formation characteristic of DVT. Thrombus-resident neutrophils were indispensable for subsequent DVT propagation. Platelets contributed to DVT progression by promoting leukocyte recruitment and stimulating neutrophil-dependent coagulation. Thus, there is a cross-talk talk between monocytes, neutrophils, and platelets responsible for the initiation and amplification of DVT and its unique clinical features (Figure 1) [18].
Figure 1: Venous thrombus formation 48h after partial (80%) inferior vena cava ligation (IVC) in two different mice strains and its prevention by enoxaparin. Note the occlusive thrombus (arrows) and the intact endothelium upstream of the ligation site (A). (B) Morphological assessment of venous thrombus 48h after flow restriction. Note the typical white (w) and red (r) cell enriched regions and the typical r/w layering (from ref. 18).
More recent work of this group has identified blood-derived “high-mobility group box 1”protein (HMGB1), a prototypical mediator of sterile inflammation in VTE, and has considered this factor, to be a master regulator of the prothrombotic cascade involving platelets and myeloid leukocytes [26]. Finally, HMGB1 has recently been identified as a new salicylate- binding protein. Salicylate at low to medium concentrations (100 μM) was found to suppress both the chemoattractant activity of fully reduced HMGB1 and the increased expression of proinflammatory cytokine genes and cyclooxygenase 2 (COX-2) induced by disulfide HMGB1 [27]. These are exciting new findings which provide fresh insights in platelet-mediated and aspirin-sensitive antithrombotic/antiinflammatory eventsin DVT.
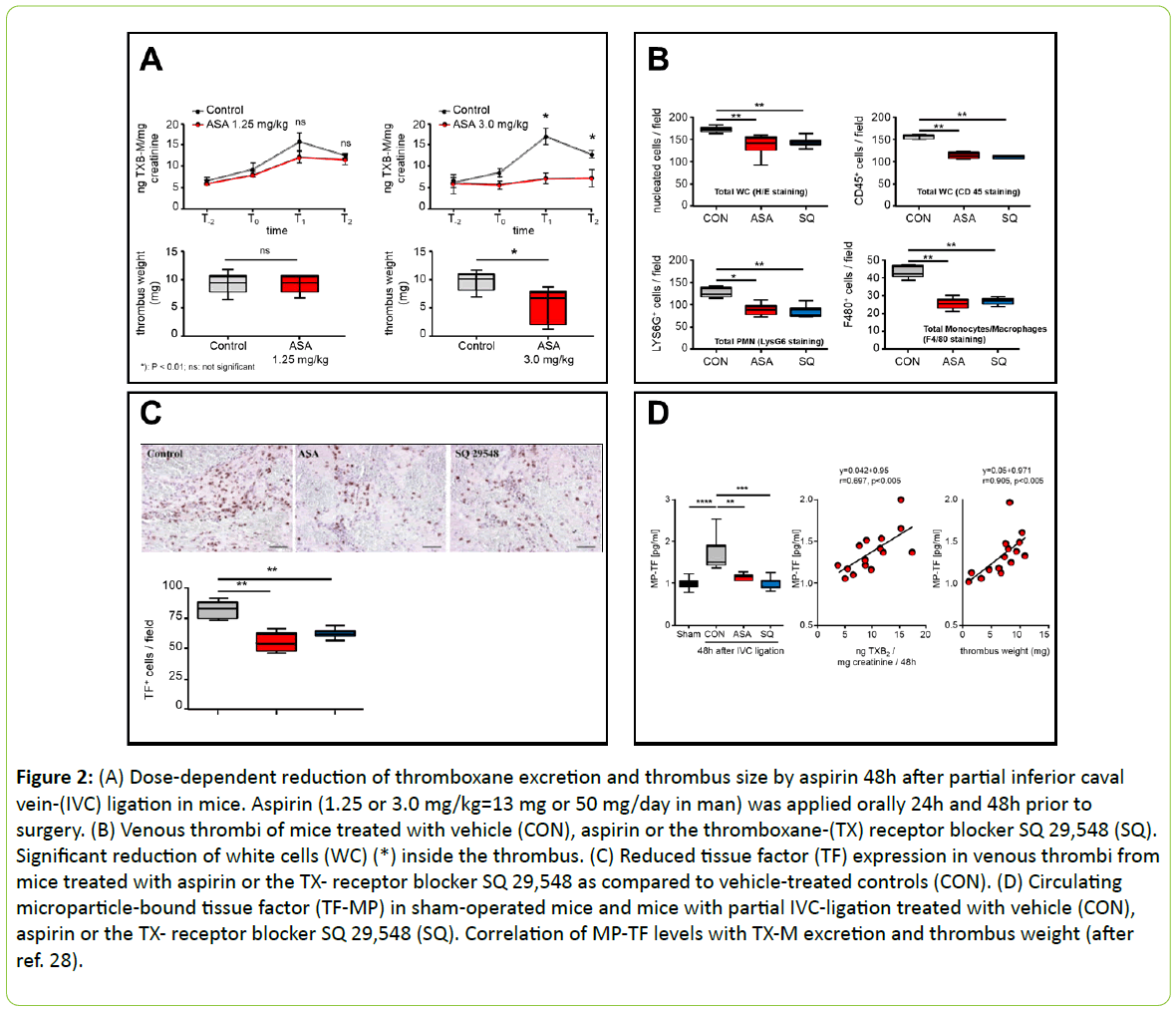
Direct experimental evidence for the involvement of plateletderived thromboxane in these actions was provided recently by an Italian group, using the same experimental model of VTE in mice [28]. ASA (3 mg/kg, daily for 2 days) reduced thrombus size, the amounts of tissue factor activity in plasma microvesicles (TF-MP) and the levels of 2,3-dinor thromboxane B2 (TXB-M) in urine. The thrombus size was positively correlated with both TFMP activity and TXB-M. In addition, a positive correlation was observed between TF-MP activity and TXB-M. A reduced number of neutrophils and monocytes and of TF-positive cells accompanied by a lower amount of fibrin and neutrophil extracellular traps (NETs) were also found in thrombi of ASAtreated mice. Similar results were obtained when mice were treated 24 hours before vessel ligation with SQ 29,548, a selective thromboxane receptor antagonist. In conclusion, ASA, suppressing TXA2 formation, prevents macrophage and neutrophil activation and markedly reduces thrombus size by a mechanism most likely dependent of the inhibition of TF activity and formation of “neutrophil extracellular traps” (NETs) (Figures 2A-D) [28].
Figure 2: (A) Dose-dependent reduction of thromboxane excretion and thrombus size by aspirin 48h after partial inferior caval vein-(IVC) ligation in mice. Aspirin (1.25 or 3.0 mg/kg=13 mg or 50 mg/day in man) was applied orally 24h and 48h prior to surgery. (B) Venous thrombi of mice treated with vehicle (CON), aspirin or the thromboxane-(TX) receptor blocker SQ 29,548 (SQ). Significant reduction of white cells (WC) (*) inside the thrombus. (C) Reduced tissue factor (TF) expression in venous thrombi from mice treated with aspirin or the TX- receptor blocker SQ 29,548 as compared to vehicle-treated controls (CON). (D) Circulating microparticle-bound tissue factor (TF-MP) in sham-operated mice and mice with partial IVC-ligation treated with vehicle (CON), aspirin or the TX- receptor blocker SQ 29,548 (SQ). Correlation of MP-TF levels with TX-M excretion and thrombus weight (after ref. 28).
Clinical trials with aspirin vs. warfarin-type anticoagulants and heparins in prevention of VTE
Similar to experimental studies, epidemiological data also suggest a relationship between platelet reactivity and VTE [29]. Actual studies in surgical intensive care unit patients have shown an aspirin-sensitive contribution of platelets to (venous) thrombogenesis, clot strength and fibrin formation [30]. These and other data suggest a platelet-related connection between arterial and venous thromboembolism. For these reasons, it has to be expected that antiplatelet agents, such as aspirin, may also be an useful option not only to prevent (retard) arterial but also venous thrombus formation.
According to an early meta-analysis of the Antiplatelet Trialists' Collaboration, aspirin reduced the number of VTE and symptomatic PE highly significantly but was less effective than heparins or cumarins [31]. As a consequence, aspirin was considered to be of only minor if any importance as a preventive of VTE. More recent data have challenged this view, including secondary prevention of recurrent VTE, and extended postsurgical VTE-prophylaxis. The last process has mainly been studied in orthopedic bone and joint (knee, hips) surgery. Therefore, the following discussion on primary prevention is focused on this type of orthopedic surgery.
Warfarin or heparin-type anticoagulants have dominated the scene for a long time [32] and still do, although now with some restrictions (see below). In a review of articles published between 1998-2007, Sharrock and colleagues [33] determined the incidence of all-cause mortality and pulmonary embolism in a total of 15,839 patients undergoing total joint arthroplasty with different thromboprophylaxis protocols. They found the 3 month mortality of several standard anticoagulant-treated groups being about twofold higher than a multimodal VTEprophylaxis including aspirin and regional anaesthesia [33].
In a large, retrospective cohort study in 307 US hospitals, a total of 93,840 patients, undergoing primary knee arthroplasty received guideline-directed treatment with warfarin (51,923), LMWH/Fondaparinux (37,198) or (multimodal) aspirin (4,719). During the observation period of 2 years, the lowest rates of VTE occurred in the aspirin group (2.3%) as opposed to warfarin (4.0%) (P=0.01) or LMWH/fondaparinux (3.1%) (n.s.).
Surgical site bleeding initially tended to be higher with the injectable agents and warfarin (P=0.01), but the adjusted analysis found no differences. There were no differences in risk of bleeding, infection or mortality after adjustment. The conclusion was that aspirin when used in conjunction with other clinical care protocols may be an effective preventive for VTE in certain patients undergoing knee arthroplasty [34].
A limitation of the study was that patients in the aspirin group had a significantly lower base-line risk for VTE (P=0.01). In addition, the authors critically commented that only 5% of their study patients were treated with aspirin as opposed to 40% with injectable drugs (heparins) and 55% with cumarin-type anticoagulants [34].
There are two even larger registry trials from the UK in patients with hip- or knee replacement, respectively which also indicated beneficial effects for aspirin. A total of 108,000 and 157,000 patients were included in each register. Twenty one and 23% of them received aspirin and the remaining LMWH. During a postsurgical observation period of 3 months there were no significant differences between the incidences of PE, severe bleeding or mortality between the two treatment regimens [35,36].
Another, most interesting trial compared aspirin with warfarin in a retrospective obervational cohort study on 3,156 patients subjected to total joint athroplasty. A total of 1,456 patients received aspirin (325 mg bid) and 1700 warfarin (INR 1.8 – 2.0) for 6 weeks after the operative procedure.
Efficacy endpoint was pulmonary embolism (PE), safety endopoint periprosthetic joint infections. There was one case of PE in the aspirin group as opposed to 5 cases in the warfarin group (0.1% vs. 0.3%, P<0.001). The incidence of perioperative joint infections was 0.4% in the aspirin group and 1.5% in the warfarin group (P<0.001). The conclusion was that the use of aspirin instead of warfarin for VTE prophylaxis reduced the risk of periprosthetic joint infections following hip or knee replacement [37].
Finally, there is another retrospective observational trial, studying aspirin vs. warfarin for prevention of VTE in 2,997 patients with revision total joint arthroplasty. There were less VTE: 0.56% vs. 1.75% but more bleedings: 1.5% vs. 0.4% in the warfarin-treated patients as compared to aspirin.
The number of surgical site infections was not different. The authors concluded that aspirin in these patients might be more effective than warfarin and is associated with a lower rate of complications [38].
Slightly different, but in the direction also beneficial results with respect to aspirin, were obtained in an AAHKS-awarded pooled analysis of 14 prospective randomized trials on VTE prophylaxis in more than 33,000 patients with hip- and kneesurgery, including those from the large Pulmonary Embolism Trial [39]. The hypothesis was that aspirin will cause less operative site bleedings without increasing the risk for thromboembolic events.
The frequency of clinically relevant, symptomatic VTE and PE in aspirin-treated patients was not different from that of patients treated with warfarin or heparins (LMWH, fondaparinux). However, the relative risk of periprocedural bleedings at the operation site was 4.9fold (warfarin), 6.4fold (LMWH) and 4.2 fold (fondaparinux) higher than that after aspirin (=1). The author concluded that these data support the use of aspirin for prophylaxis of VTE after major orthopaedic surgery [40].
Another prospective randomized trial in patients with hip or knee arthroplasty showed that aspirin was inferior to warfarinbased prevention of VTE in patients at standard risk of VTE with respect to symptomatic VTE (4.6% vs. 0.7%) and PE (7.9% vs. 1.2%).
No differences were seen in patients at elevated vascular risk and there were also no differences in overall mortality [41]. It should, however, be noted that the administration of aspirin in No differences were seen in patients at elevated vascular risk and there were also no differences in overall mortality [41]. It should, however, be noted that the administration of aspirin in British Biomedical Bulletin Vol.5 No.1:297 2017 4 This article is available from: https://www.imedpub.com/british-biomedical-bulletin/ this study was quite unusual: initially 600 mg rectal, followed by 325 mg oral bid.
The currently last, but also first available prospective randomized study on extended use of aspirin vs. LMWH for primary prevention of VTE in patients after hip arthroplasty, was the Canadian “Extended Prophylaxis Comparing Low Molecular Weight Heparin to Aspirin in Total Hip Arthroplasty” (EPCAT) Study: This was a comparison for noninferiority of aspirin vs. dalteparin in patients subjected to unilateral total hip arthroplasty. A total of 778 patients received initially for 10 days dalteparin sc. and were afterwards randomized for another 28 days to either aspirin (81 mg/day+dalteparin-placebo sc.) or dalteparin (5000 U/day sc. + aspirin-placebo tablet).
1.3% of the dalteparin group and 0.3% of the aspirin group suffered a VTE. Aspirin was noninferior (P=0.01) but not superior to dalteparin (P=0.22). Clinically significant bleedings occurred at 1.3% in the dalteparin and 0.5% in the aspirin group. The net event rate (VTE plus bleeding was 0.8% for aspirin and 2.5% for dalteparin with a tendency in favour of aspirin (P=0.091). Unfortunately, the study had to be stopped prematurely because of difficulties with patient recruitment.
However, at this time point noninferiority was already reached. The conclusion was that extended prophylaxis for 28 days with aspirin is noninferior and as safe as dalteparin for the prevention of VTE, suggesting that aspirin may be considered a reasonable alternative for extended thromboprophylaxis after hip replacement [9]. This study was the first to document a noninferiority of aspirin vs. LMWH in a combined therapeutic approach for prevention of primary VTE.
Clinical trials with NOAC vs. aspirin in prevention of VTE
A first prospective randomized trial on aspirin (100 mg/day), NOAC (rivaroxaban) and LMWH in prevention of postsurgical VTE is also available. A total of 324 patients with knee replacement was treated for 2 weeks and followed for 4 weeks afterwards. Rivaroxaban-treated patients showed the lowest incidence of DVT (2.9%) as opposed to heparin (12.4%) and aspirin (16.4%).
There were no differences in overall outcome between aspirin and LMWH. However, there was an increased postoperative blood loss and wound complications with rivaroxaban. The conclusion was that clinicians using rivaroxaban for anticoagulant treatment should closely monitor the changes in the hemoglobin level and wound healing and promptly supplement blood volume and provide other symptomatic and supportive treatments if necessary [42].
Aspirin in long-term prevention of recurrent VTE
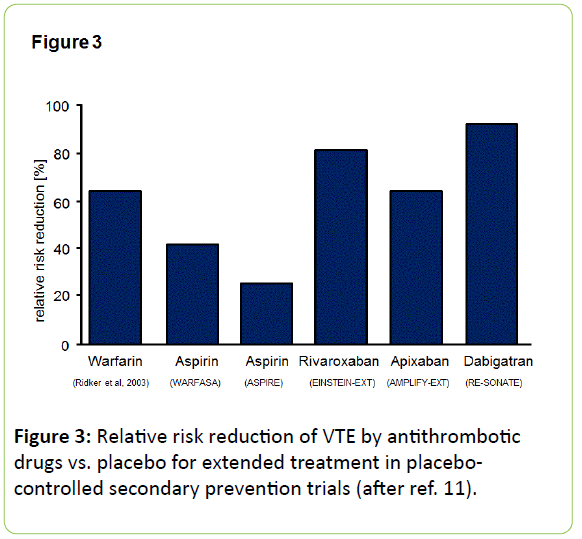
Actual guidelines recommend anticoagulants after a primary DVT or PE for several weeks or months [43]. This long-term prevention of recurrent VTE is the domain of oral anticoagulants, now also of NOAC. An overview on therapeutic efficacy of anticoagulants vs. aspirin in extended treatment in placebo-controlled secondary prevention trials is shown as Figure 3.
Two prospective randomized, placebo-controlled trials have recently studied, whether extended treatment with low-dose aspirin after the end of guideline-directed anticoagulation will have a beneficial effect on prevention of recurrent VTE: The “Warfarin and Acetylsalicylic Acid” (WARFASA) [10] and the “Aspirin to Prevent Recurrent Venous Thromboembolism“ (A SPIRE)-study [44].
In the WARFASA-trial there was a reduction of recurrent VTE after withdrawal of oral anticoagulants by almost 41% without increase in major bleedings. In the ASPIRE-trial, aspirin only tended to reduce the incidence of recurrent VTE from 6.5% auf 4.8% (HR 0.74; 95%KI: 0.52-1.05) which was not significant (P=0.09).
However, aspirin reduced the rate of a prespecified secondary composite endpoint (VTE, myocardial infarction, stroke, cardiovascular death) from 8.0% per year to 5.2% per year (HR 0.66; 95% CI 0.48–0.92), i.e. by 34%.
This was mainly driven by an about 50% reduction by aspirin of arterial thrombotic events: 10-19 (P=0.01). There were no significant differences in major or clinically relevant bleedings: Placebo (0.6% per year) vs. aspirin (1% per year). Taken together, there was a reduction in venous and arterial thromboembolism by one third (P=0.002).
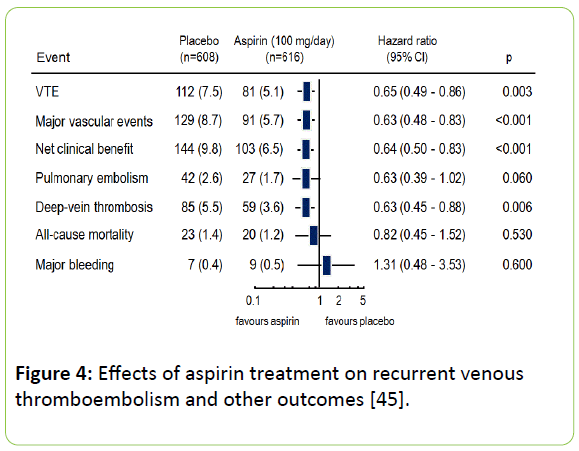
A combined evaluation of the two studies, using standardized evaluation criteria, the INSPIRE trial, has confirmed these findings [45]. Aspirin treatment after the end of guide-line directed anticoagulants reduced the risk of recurrent VTE at 30 months in comparison to placebo by 42% (HR:0.58; 95% CI: 0.40-0.85; p=0.005) at an unchanged number of severe bleedings: 0.4% per year for aspirin and 0.5% per year for placebo (Figure 4).
Current status and Outlook
The major limitation of available studies on anticoagulants vs. aspirin in VTE prevention is their uncontrolled nature – despite of the huge total number of patients included. There are only very few randomized trials, hosever, with the exception of the Pulmonary Embolism study, in only small numbers of patients. In this context, cross-comparisons might lead to misleading information because of bias, for example patient selection bias as shown recently by comparing the clinical outcome in two VTE-prevention trials: The EINSTEIN-DVT/PE and AMPLIFY trials which yielded different results in dependency on patient selection and treatment duration [46]. Despite the fact that NOAC appear to be more potent drugs in extended prevention of VTE as opposed to aspirin (cf. Figure 4), they might also cause more bleedings [6]. Interestingly, despite of comparable efficacy, there might be significant differences between the different NOAC, favoring apixaban as opposed to rivaroxaban and dabigatran [5,8]. However, there is also the question whether “one size [of drugs] fits all [patients]”. Clearly there is an urgent need for more randomized controlled head-to-head comparions of aspirin with NOAC.
The actual guidelines of ACCP from 2012 contain for the first time a special chapter regarding prevention of VTE in orthopaedic surgery. This contains now also aspirin together with anticoagulants as another therapeutic option. The American Academy of Orthopaedic Surgeons (AAOS) had already in 2007 recommended aspirin for the same indication as alternative to anticoagulants. The actual guidelines from 2011 recommend “pharmacological agents” without more detailed classification which probably includes aspirin. Thus, both societies now accept aspirin as a pharmacological alternative for prevention of VTE despite of their different definitions of efficacy: only symptomatic or fatal PE (AAOS) but no DVTs vs. all DVTs and PE (ACCP) [47]. European societies, such as the British National Institute of Health and Care Excellence (NICE) as well as the German AWMF are more restrictive and refuse aspirin as a pharmacological option for prevention and post-hospital treatment of VTE. However, they do recommend NOAC for both situations in addition to LMWH [48]. Two studies, the EINSTEINCHOICE trial (clinicaltrials.gov. NCT02064439) comparing aspirin with two doses of rivaroxaban in prevention of recurrent VTE [49] as well as the EPCAT-2 trial (clinicaltrials.gov.NCT 017220108) are underway.
The new experimental data on the natural history of VTE have proivided fresh insights into the pathophysiology of the disease and might help to select the most appropriate prevention programme for VTE. One recent metaanalysis suggests that over 90% of patients undergoing joint arthroplasty could safely receive aspirin as an anticoagulant, while a validated risk profile can be used to detect those at higher risk for VTE and in need of more aggressive treatment [50]. Though this might be a too optimistic view, another recent study comparing aspirin with warfarin in primary prevention of VTE has demonstrated that the use of an approproiate risk stratification protocol will avoid more aggressive anticoagulation in favor of aspirin in 70% of patients [51]. Thus, aspirin in a combined approach may be sufficient for many patients undergoing joint surgery. It is now the task of clinical studies to define an optimum treatment protocol for primary and secondary VTE prophylaxis. The individual risk profile of the patient will play a significant role, including (advanced) atherosclerosis with enhanced risk for atherothrombotic vessel occlusions, requiring aspirin treatment. Factor Xa antagonists might have an indirect inhibitory effect on platelet function via inhibition of thrombin formation but are no directly acting antiplatelet agents.
Acknowledgement
The authors are grateful to Petra Rompel for her competent assistance in preparing the figures.
Funding
None
References
- Turpie AG, Esmon C (2011) Venous and arterial thrombosis--pathogenesis and the rationale for anticoagulation. Thromb Haemost 105: 586-596.
- Wolowacz SE, Roskell NS, Plumb JM, Caprini JA, Eriksson BI (2009) Efficacy and safety of dabigatran etexilate for the prevention of venous thromboembolism following total hip or knee arthroplasty. A meta-analysis. Thromb Haemost 101: 77-85.
- Eriksson BI, Orris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, et al. (2008) Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med 358: 2765-2775.
- Venker BT, Ganti BR, Lin H, Lee ED, Nunley RM, et al. (2016) Safety and efficacy of new anticoagulants for the prevention of venous thromboembolism after hip and knee arthroplasty: a meta-analysis. J Arthroplasty 32: 645-652
- Cohen AT, Hamilton M, Bird A, Mitchell SA, Li S, et al. (2016) Comparison of the non-vka oral anticoagulants apixaban, dabigatran, and rivaroxaban in the extended treatment and prevention of venous thromboembolism: systematic review and network meta-analysis. PLoS One. 11: e0160064.
- Sardar P, Chatterjee S, Mukherjee D (2013) Efficacy and safety of new oral anticoagulants for extended treatment of venous thromboembolism: systematic review and meta-analyses of randomized controlled trials. Drugs 73: 1171-1182.
- Hirschl M, Kundi M (2014) New oral anticoagulants in the treatment of acute venous thromboembolism - a systematic review with indirect comparisons. Vasa 43: 353-364.
- Mantha S, Ansell J (2015) Indirect comparison of dabigatran, rivaroxaban, apixaban and edoxaban for the treatment of acute venous thromboembolism. J Thromb Thrombolysis 39: 155-165.
- Anderson DR, Dunbar MJ, Bohm ER, Belzile E, Kahn SR, et al. (2013) Aspirin versus low-molecular-weight heparin for extended venous thromboembolism prophylaxis after total hip arthroplasty: a randomized trial. Ann Intern Med 158: 800-806.
- Becattini C (2012) Aspirin for preventing the recurrence of venous thromboembolism. N Engl J Med 366: 1959-1967.
- Cohen AT, Markham J, Granziera S, Imfeld S (2015) The use of aspirin for primary and secondary prevention in venous thromboembolism and other cardiovascular disorders. Thromb Res, 135: 217-225.
- Marik PE, Cavallazzi R (2015) Extended anticoagulant and aspirin treatment for the secondary prevention of thromboembolic disease: a systematic review and meta-analysis. PLoS One 10: e0143252.
- Green D (2009) Risk of future arterial cardiovascular events in patients with idiopathic venous thromboembolism. Hematology Am Soc Hematol Educ Program 259-266.
- Prandoni P, Bilora F, Marchiori A, Bernardi E, Petrobelli F, et al. (2003) An association between atherosclerosis and venous thrombosis. N Engl J Med 348: 1435-1441.
- Schrör K (2016) Clinical applications of aspirin, in Acetylsalicylic acid, Wiley-VCH Verlag GmbH and Co. KGaa, Weinheim pp: 265-446.
- Undas, A, Brummel-Ziedins, K, Mann KG (2014) Why does aspirin decrease the risk of venous thromboembolism? On old and novel antithrombotic effects of acetyl salicylic acid. J Thromb Haemost 12: 1776-1787.
- Bovill EG, A van der Vliet (2011) Venous valvular stasis-associated hypoxia and thrombosis: what is the link? Annu Rev Physiol 73: 527-545.
- Von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209: 819-835.
- Sevitt S (1970) Thrombosis and embolism after injury. J Clin Pathol Suppl (R Coll Pathol) 4: 86-101.
- Heemskerk JW, Bevers EM, Lindhout T (2002) Platelet activation and blood coagulation. Thromb Haemost 88: 186-193.
- Wallen NH, Ladjevardi M (1998) Influence of low- and high-dose aspirin treatment on thrombin generation in whole blood. Thromb Res 92: 189-194.
- Szczeklik A, Krzanowski M, Góra P, Radwan J, et al. (1992) Antiplatelet drugs and generation of thrombin in clotting blood. Blood 80: 2006-2011.
- Undas A, Undas R, Musiał J, Szczeklik A, et al. (2000) A low dose of aspirin (75 mg/day) lowers thrombin generation to a similar extent as a high dose of aspirin (300 mg/day). Blood Coagul Fibrinolysis 11: 231-234.
- Heestermans M, Salloum-Asfar S, Salvatori D, Laghmani el H, Luken BM, et al. (2016) Role of platelets, neutrophils, and factor XII in spontaneous venous thrombosis in mice. Blood 127: 2630-2637
- Herbert JM, Bernat A, Maffrand JP (1992) Importance of platelets in experimental venous thrombosis in the rat. Blood 80: 2281-2286.
- Stark K, Philippi V, Stockhausen S, Busse J, Antonelli A, et al. (2016) Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 128: 2435-2449.
- Choi HW, Tian M, Song F, Venereau E, Preti A, et al. (2015) Aspirin's active metabolite salicylic acid targets high mobility group box 1 to modulate inflammatory responses. Mol Med 21: 526-535.
- Tarantino E, Amadio P, Squellerio I, Porro B, Sandrini L, et al. (2016) Role of thromboxane-dependent platelet activation in venous thrombosis: Aspirin effects in mouse model. Pharmacol Res 107: 415-425.
- Braekkan SK, Mathiesen EB, Njølstad I, Wilsgaard T, Størmer J, et al. (2009) Mean platelet volume is a risk factor for venous thromboembolism: the Tromso Study, Tromso, Norway. J Thromb Haemost 8: 157-162.
- Harr JN, Moore EE, Chin TL, Ghasabyan A, Gonzalez E, et al. (2013) Platelets are dominant contributors to hypercoagulability after injury. J Trauma Acute Care Surg 74: 756-762.
- Antiplatelet T (1994) Collaboration, Collaborative overview of randomised trials of antiplatelet therapy. III. Reduction in venous thrombosis and pulmonary embolism by antiplatelet prophylaxis among surgical and medical patients. Br Med J 308: 235-246.
- Brotman DJ (2003) Low-intensity versus conventional-intensity warfarin for prevention of recurrent venous thromboembolism. N Engl J Med 349: 2164-2167.
- Sharrock NE, Gonzalez Della Valle A, Go G, Lyman S, Salvati EA, et al. (2008) Potent anticoagulants are associated with a higher all-cause mortality rate after hip and knee arthroplasty. Clin Orthop Relat Res 466: 714-721.
- Bozic KJ, Vail TP, Pekow PS, Maselli JH, Lindenauer PK, et al. (2010) Does aspirin have a role in venous thromboembolism prophylaxis in total knee arthroplasty patients? J Arthroplasty 25: 1053-1060.
- Jameson SS, Baker PN, Charman SC, Deehan DJ, Reed MR, et al. (2011) The effect of aspirin and low-molecular-weight heparin on venous thromboembolism after knee replacement: a non-randomised comparison using National Joint Registry Data. J Bone Joint Surg Br 94: 914-918.
- Jameson SS, Charman SC, Gregg PJ, Reed MR, van der Meulen JH, et al. (2011) The effect of aspirin and low-molecular-weight heparin on venous thromboembolism after hip replacement: a non-randomised comparison from information in the National Joint Registry. J Bone Joint Surg Br 93: 1465-1470.
- Huang, R, Buckley PS, Scott B, Parvizi J, Purtill JJ, et al. (2015) Administration of aspirin as a prophylaxis agent against venous thromboembolism results in lower incidence of periprosthetic joint infection. J Arthroplasty 30: 39-41
- Deirmengian G, Heller S, Smith EB, Maltenfort M, Chen AF, et al. (2016) Aspirin can be used as prophylaxis for prevention of venous thromboembolism after revision hip and knee arthroplasty. H Arthroplasty, 31: 2237-2240.
- Rodgers A (2000) Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary embolism prevention (PEP) trial. Lancet 355: 1295-1302.
- Brown GA (2009) Venous thromboembolism prophylaxis after major orthopaedic surgery: a pooled analysis of randomized controlled trials. J Arthroplasty 24: 77-83.
- Committee IJRCW (2012) A prospective comparison of warfarin to aspirin for thromboprophylaxis in total hip and total knee arthoplasty. J Arthroplasty 27: 1-9 e2.
- Zou Y, Tian S, Wang Y, Sun K (2014) Administering aspirin, rivaroxaban and low-molecular-weight heparin to prevent deep venous thrombosis after total knee arthroplasty, in Blood Coagul Fibrinolysis 25: 660-664
- Warkentin TE (2012) Aspirin for dual prevention of venous and arterial thrombosis. N Engl J Med 367: 2039-2041.
- Brighton TA, Eikelboom JW, Mann K, Mister R, Gallus A, et al. (2012) Low-dose aspirin for preventing recurrent venous thromboembolism. N Engl J Med 367: 1979-1987.
- Simes J, Becattini C, Agnelli G, Eikelboom JW, Kirby AC, et al. (2014) Aspirin for the prevention of recurrent venous thromboembolism: the INSPIRE collaboration. Circulation 130: 1062-1071.
- Beyer-Westendorf J, Lensing AW, Arya R, Bounameaux H, Cohen AT, et al. (2016) Choosing wisely: The impact of patient selection on efficacy and safety outcomes in the EINSTEIN-DVT/PE and AMPLIFY trials. Thromb Res 149: 29-37.
- Stewart DW, JE Freshour (2013) Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother 47: 63-74.
- NICE (2015) Venous thromboembolism: reducing the risk for patients in hospital. Clinical guideline [CG92].
- Weitz JI, Bauersachs R, Beyer-Westendorf J, Bounameaux H, Brighton TA, et al. (2015) Two doses of rivaroxaban versus aspirin for prevention of recurrent venous thromboembolism. Rationale for and design of the EINSTEIN CHOICE study. Thromb Haemost 114: 645-650.
- Dalury D, Lonner J, Parvizi J (2015) Prevention of venous thromboembolism after total joint arthroplasty: aspirin is enough for most patients. Am J Orthop (Belle Mead NJ) 44: 59-60.
- Nam D, Nunley RM, Johnson SR, Keeney JA, Clohisy JC, et al. (2016) The effectiveness of a risk stratification protocol for thromboembolism prophylaxis after hip and knee arthroplasty. J Arthroplasty 31: 1299-1306.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences