ISSN : 2634-7814
Journal of Chemical Biology & Pharmaceutical Chemistry
A Systematic Review of the Comparison of Three Medicinal Licorices, Based on Differences of the Types and Contents about Their Bioactive Components
Yong-Jiao Zhang, Hai-Bin Gong and Hai-Liang Zhu*
State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210023, China
- *Corresponding Author:
- Hai-Liang Zhu
State Key Laboratory of Pharmaceutical Biotechnology
Nanjing University, Nanjing 210023, China
Tel: 025-89682572
E-mail: zhuhl@nju.edu.cn
Received date: February 02, 2018; Accepted date: February 16, 2018; Published date: February 23, 2018
Citation: Zhang YJ, Gong HB, Zhu HL (2018) A Systematic Review of the Comparison of Three Medicinal Licorices, Based on Differences of the Types and Contents about Their Bioactive Components. J Chem Biol Pharm Chem Vol.1 No.1:03
Copyright:© 2018 Zhang YJ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Licorice, derived from the roots and rhizomes of Glycyrrhiza genus, is widely distributed all over the world. In China, three Glycyrrhiza species, G. uralensis, G. glabra, and G. inflata, are used as licorice without discrimination according to the 2015 edition of the Chinese Pharmacopoeia. Triterpenoid saponins, flavonoids and polysaccharides are considered as bioactive ingredients of licorice. The component properties, types and contents of these chemical compounds, vary significantly in different species and different licorice species have species-specific markers respectively, such as the contents of major flavonoids (liquiritin, liquilitigenin and isoliquiritin) in G. uralensis are obviously higher than in G. glabra and glycycoumarin only exists in G. uralensis. To some extent variation in active constituent could affect the therapeutic effects and safety of licorice, thus licorice used for medicinal purposes should be discriminated and chemically standardized according to those component properties in order to promote its reasonable application.
In this review, we describe differences of the types and contents of chemical composition among three licorice species. The result of this review is intended to provides the basis for the accurate identification and reasonable use of licorice.
Keywords
Licorice; Triterpenoid saponins; Flavonoids; Polysaccharides; Species-specific markers
Introduction
Licorice (Gancao in Chinese), one of the oldest and most widely used herbals in the world, has been used by human beings for over 4000 years. As a popular herbal medicine, licorice has been extensively used in the at least 70% of traditional Chinese medicine (TCM) for the effects of tonifying spleen and replenishing qi, eliminating phlegm and relieving cough, clearing away heat, detoxifying and used for the treatment of coughs, peptic ulcers, inflammation, hepatic disease, viral infections, cancer, and other ailments [1-3]. It is also widely used in food industry and cosmetics industry due to its sweet taste, whitening and anti-inflammatory activity. Modern phytochemical studies have demonstrated that the main active substances of licorice are triterpenoid saponins, flavonoids and polysaccharides, which have a variety of pharmacological activities, including anti-inflammatory, antibacterial, antiviral, antiulcer, antioxidative, antiallergic, anticancer, antidiabetic, anti-depressive, hepatoprotective, immunomodulatory and cardioprotective effects [4-8].
In China, G. uralensis, G. glabra, and G. inflata are considered as being equivalent and are combined and utilized as licorice without discrimination according to the 2015 edition of the Chinese Pharmacopoeia. It is difficult to identify these licorice species accurately based on their root or rhizome morphology [9]. But the contents of theses active ingredients may vary significantly due to interspecific differences of licorice, which thus affect their quality, safety and therapeutic effects. In addition, licorices are increasingly being used in the fields of medicine, food, and cosmetics. Therefore, comprehensive quality control and comparative constituent properties to these licorice species are critical to ensure their efficacy and safety.
Quality control analyses in licorice mostly target single compound examination, of which glycyrrhizic acid is the most frequently examined compound. However, with an increasing demand for accuracy and consistency of phytomedicine bioactivity, and the fact that not all pharmacological effects may originate from only one sole constituent, single targeted constituent is an insufficient tool for standardization of licorice [10]. Recently, flavonoids and polysaccharides in licorice are also determined to assess and control the quality of licorice because of their potent activities [11-24].
Numerous studies have established analytical methods to separate and quantify these active ingredients in licorice samples. Although, prior research has attempted to describe the dissimilar composition of the licorice species, these reports most simultaneously compared only one or two classes of ingredients among the triterpenoid saponins, flavonoids and polysaccharides or only simultaneously determined several active chemical compounds [9,10,25,26]. These have been no reports of systematic and comprehensive review for tree active ingredients (triterpenoid saponins, flavonoids, polysaccharides).
In this review, we describe the variations in constituent properties (types and contents of bioactive ingredients) of licorice species derived from G. uralensis, G. glabra and G. inflata to improve the comprehension of chemical characteristics of licorice and provides the basis for its quality control and reasonable use.
Triterpene saponins
Triterpene saponins, represented by glycyrrhizic acid and presented as glucuronides mostly, are the major characteristic ingredients in licorice and exhibit extensive biological activities including hepatoprotective, anti-cancer, antiviral, antiinflammatory [27-29]. However, overconsumption of licorice may result in side effects like sodium and water retention, hypertension, hypokalemia, and suppression of the reninaldosterone system [30,31]. Therefore, the saponins are of great importance for the efficacy and safety in clinical use of licorice, which makes their analysis of significant interest.
A number of studies have attempted to describe the dissimilarities of the triterpene species in the Glycyrrhiza species. For example, Tao W, et al. measured 10 triterpenoid saponins in 82 batches of three licorice species, collected from their main production regions in China and revealed that both the types and contents of the triterpene saponins were significantly different among species [9]. Song W, et al. measured 151 secondary metabolites of three medicinal Glycyrrhiza species and noted that the contents of 24 measured triterpenoids in G. inflata are higher than which in other two species [32].
Glycyrrhizin
Glycyrrhizin, a saponin of the pentacyclic triterpene derivative of the oleanane type, is the principal saponin in licorice and generally known for its numerous medicinal benefits at appropriate doses such as anti-inflammatory, anti-allergic, antiviral, hepatoprotective, antiulcer, antiallergy [33,34]. Following hydrolysis, it releases two molecules of D-glucuronic acid and the aglycone glycyrrhetic acid which has been found to exhibit anti-inflammatory, anti-allergic, anti-filarial, anti-cancer, neuroprotective effects [35-37]. In regard to quality control analysis of licorice, glycyrrhizin is often considered as the most frequently monitored metabolite via HPLC [38]. Based on these considerations, the content of glycyrrhizin is a very important ingredient in the dietary and medicinal use of licorice.
Song W, et al. reported that glycyrrhizin was evenly distributed in the three species (40.5 ± 29.1, 49.3 ± 15.6, and 40.6 ± 26.4 mg/g in G. uralensis, G. inflata, and G. glabra, respectively [32], which are identified from their genetic information. Kondo K, et al. also reported that there was no significant discrepancy among the three species regarding the contents of glycyrrhizin [39].
But some studies suggested that the content of glycyrrhizin is different in three licorice species. For example, Tao W, et al. reported that the average ratios of glycyrrhizin in total content of 10 investigated triterpene saponins were 76.8% for G. inflata, 83.29% for G. glabra, and 51.10–56.15% for G. uralensis [9]. Rizzato G, et al. analyzed ten different commercial samples of Glycyrrhiza dried roots (samples of G. glabra from Europe, samples of G. uralensis and G. inflata from China) and indicated that glycyrrhizin showed a significantly higher signal intensity in G. glabra than in Chinese licorice (G. uralensis and G. inflata), in which glycyrrhizin did not demonstrated any substantial differences [40]. Whilst Farag MA, et al. noted that the content of glycyrrhizin in licorice root samples derived from different samples were various and Chinese G. inflata roots contained the highest levels of glycyrrhizin, lowest levels were found in G. glabra (Syria) [10].
The results are inconsistent, which may because the existences of hybrids among Glycyrrhiza species [39] and the content of glycyrrhizic acid is affected not only by their different species but also by geographic area, state of plant maturity, environmental conditions, harvesting and processing [41]. Therefore, glycyrrhizin is inadequate for species distinction, although it is used for the quality control of licorice.
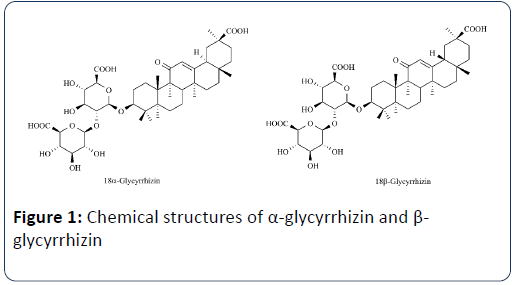
Glycyrrhizin isomer
Glycyrrhizin exists as 2 isomers viz α-glycyrrhizin and β- glycyrrhizin with commercial glycyrrhizin products containing mainly the latter (Figure 1).
Both isomers are effective but there are dissimilarities in their biological activities and physicochemical properties. α- glycyrrhizin appears to have better lipophilicity, higher targeting, greater anti-inflammatory activity and lower incidence of adverse effects. α-glycyrrhizin has been widely used as a fourth generation of glycyrrhizic acid preparation, such as Magnesium isoglycyrrhizinate is a pure salt of α-glycyrrhizin which was developed as a new treatment for chronic hepatitis [42].
For a long time, both α-glycyrrhizin and β-glycyrrhizin were considered as total glycyrrhizin in qualitative and quantitative analysis with no separation. Recently, some studies analyzed glycyrrhizin isomer simultaneously due to the development of quality control analysis techniques.
Ying C, et al. revealed that the content of 18α-glycyrrhizin and 18β-glycyrrhizin vary significantly, as the content of 18β- glycyrrhizin was much higher than that of 18α-glycyrrhizin [43]. Yang R, et al. established an HPLC method for simultaneous contents determination of 18α-glycyrrhizic acid and 18β-glycyrrhizic acid, and compared the contents of these two triterpenoids in licorice derived from the three species. It was demonstrated that the contents of 18α-glycyrrhizic acid and 18β-glycyrrhizic acid in G. glabra were the highest, which in G. uralensis were the second and in G. inflata were the lowest. Furthermore, the content of 18α-glycyrrhizic acid was significantly correlated to the content of 18β-glycyrrhizic acid in all three species of licorice [44].
Other Triterpene Saponins
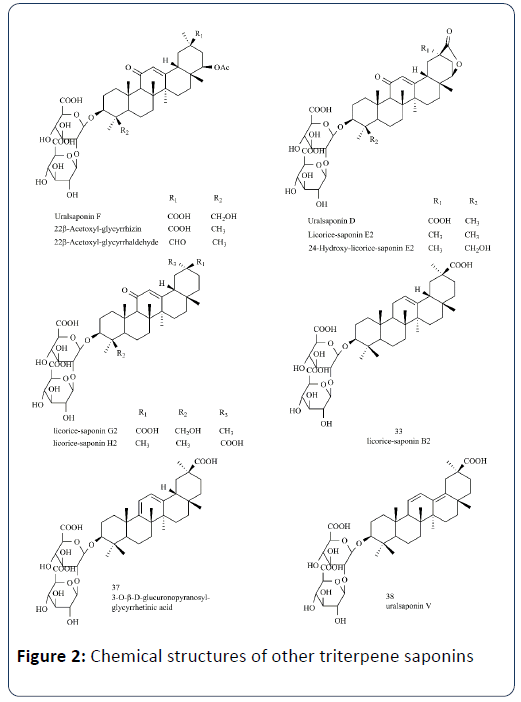
Some other triterpenoid saponins with less content have been identified and quantified with the use of more sensitive, higher precise and accurate analysis techniques (Figure 2).
Tao W, et al. revealed significant differences among species in both the type of triterpene saponins and their contents and noted that some of the major compounds may serve as chemotaxonomic markers, such as 22β-acetoxyl-glycyrrhizin (4) and licorice-saponin E2 (7) were detectable in G. uralensis and G. inflata only, while 24-hydroxy-licorice-saponin E2 (5) only in G. uralensis. And compounds uralsaponin F (2), uralsaponin D (3), and 22β-acetoxyl-glycyrrhaldehyde (8) were present in some G. inflata, but not in any G. glabra [9].
Song W, et al. found that the three Glycyrrhiza species showed distinct biosynthetic preferences. For triterpene saponins, Ering- substituted saponins (e.g., 22β-acetoxyl-glycyrrhizin) and 11-deoxy-glycyrrhizins (33, 37, 38) were relatively abundant in G. uralensis and G. inflata, respectively [32].
Rizzato G, et al. noted that three saponins–licorice saponin K2/H2 (2), licorice saponin B2 (7) and licorice saponin G2/ yunganoside K2 (8) – were higher content in G. glabra, as they were not detected with significant intensities in the other two species [40].
Flavonoids
In recent years, licorice flavonoids have become one of the hotspot of pharmacological studies for their structural diversity and important pharmacological activities of the isolated flavonoids, including chalcones, isoflavones, isoflavans, flavonones, flavanonols, isoflavenes and arylcoumarins [11]. liquiritin, isoliquiritin and liquiritigenin are considered to be the main flavonoids of licorice, exhibited a variety of biological properties including antitumor, antidepressant, neuroprotective, antioxidant and anti-inflammatory [12-15]. Licochalcone A, an oxygenated chalcone in licorice, was reported to possess antitumor, antimalarial, anti-metastatic, chemopreventive, antibacterial, and anti-spasmodic activity [16-19]. Glycycoumarin, a representative coumarin in G. uralensis, showed antithrombotic and antispasmodic activities [3]. Some isoflavans derived flavonoids in licorice, such as glabridin, licoricidin and licorisoflavan A showed antiinflammatory, antioxidative and antitumor activities [11]. The isoflavone compound dehydroglyasperin C exhibited neuroprotective effects by inducing phase II enzymes [20]. Glycyrol and glycyrin, two major licorice coumarins, showed anti-inflammatory and antihypertension activity, respectively [3]. Furthermore, Quercetin is a flavonol with strong antioxidant activity, the different content of quercetin would affect the radical scavenging activity of Glycyrrhiza species.
Numbers of studies have tried to describe the diversity of the flavonoids in the licorice species. It is reported that the types and contents of the flavonoids in different licorice species were obviously various and the content of liquiritin was higher than that of other flavonoids which was used for quality control analysis of licorice.
Liquiritin, liquilitigenin and isoliquiritin are major flavonoids in G. uralensis, G. glabra, and G. inflata. Numbers of studies have shown that the contents of those three compounds in G. uralensis are obviously higher than in G. glabra and G. inflata [25,26,32,39,45].
Zhu Z, et al. quantified 15 flavonoids in licorice and found that the contents of compounds 2, 5, 11, 12, 14 and 15 (isoliquiritin, isotrifloliol, licoricone, neoglycyrol, glycyrin and licorisoflavan A) in G. uralensis were higher than G. glabra and G. inflata while compound 9 (formononetin) were lower [25]. Qiao X, et al. revealed that the content of compounds glycycoumarin (1), dehydroglyasperin C (2), and Glycyrol (3) in G. inflata and G. glabra was lower than those in G. uralensis [3]. Fu Y, et al. noted that DTM were not detected in G. uralensis and the contents of chalcone derivatives such as licochalcone A, licochalcone B and echinatin in G. inflata were higher than in G. uralensis [11]. Rizzato G, et al. suggested that a compound belonging to the family of prenylated chalcones, kanzonol C (20), was present in G. inflata with statistically significant higher intensities than in G. glabra and G. uralensis. And a chalcone, identified as glyinflanin D or G (46) and licoflavone B (47), was found only in G. inflata and G. glabra samples [40]. Additionally, quercetin, a flavonol with strong anti-oxidant activity, was reported that presented in G. inflata with higher intensity than in G. uralensis and absented in G. glabra [10,40,46].
Song W, et al. revealed that the contents of 3-aryl-5-methoxyl coumarins (79–84) and flavonols (47–54) in G. uralensis were significantly higher than those in G. inflata or G. glabra. Likewise, G. inflata contained remarkably more abundant 2′-H chalcones (e.g., licochalcones A, C, E), and G. glabra contained more abundant 8-cyclized isoprenyl isoflavanes (e.g., glabridin and 4′-O-methylglabridin) than the other two species [32].
For flavonoid glycosides, Song W, et al. noted that the apiosylation ratio of liquiritin and isoliquiritin was significantly lower in G. uralensis (47 ± 19%) than in G. glabra and G. inflata (74 ± 13% and 82 ± 16%, P < 0.005), whereas the content ratio of flavanone glycosides (1, 4, 5) chalcone glycosides (8, 9, 10) was significantly higher in G. uralensis (2–5-fold) [32]. Li G, et al. revealed that the contents of apiosides (liquiritin apioside, isoliquiritin apioside, licuraside) in G. glabra root preparations were the highest, which in G. inflata were the second and in G. uralensis were the lowest [45].
Polysaccharide
Polysaccharide is one of the main bioactive constituents in licorice. Recently, various studies have shown that Glycyrrhiza polysaccharides had many pharmacological activities including immunoregulatory, antioxidant, antitumor, antivirus [21-24]. The determination of monosaccharides in polysaccharides is an important way to explore the quality standard of polysaccharides, study the basic information of structure, properties and structure-activity relationship of polysaccharides. Therefore, the study of polysaccharides should also be used as one of the methods to control the quality of licorice.
Wei LI, et al. used phenol-H2SO4colorimetry method measured the contents of the polysaccharide in Glycyrrhiza of different species and indicated that polysaccharide content in G. glabra was the highest, in G. inflata was second, and in G. uralensis was lowest [47]. Zhao L, et al. used phenol-sulfuric acid method to measure the polysaccharide content in different Glycyrrhiza rootspecies under the same cultivated environment and got the same result, polysaccharide content in G. glabra was the highest [48].
Wei LI, et al. showed that the monosaccharide composition of polysaccharide in different Glycyrrhiza was the same, but the proportion of monosaccharide (ManGalUAGlcGalAra) was greatly different [49].
Species-specific Markers
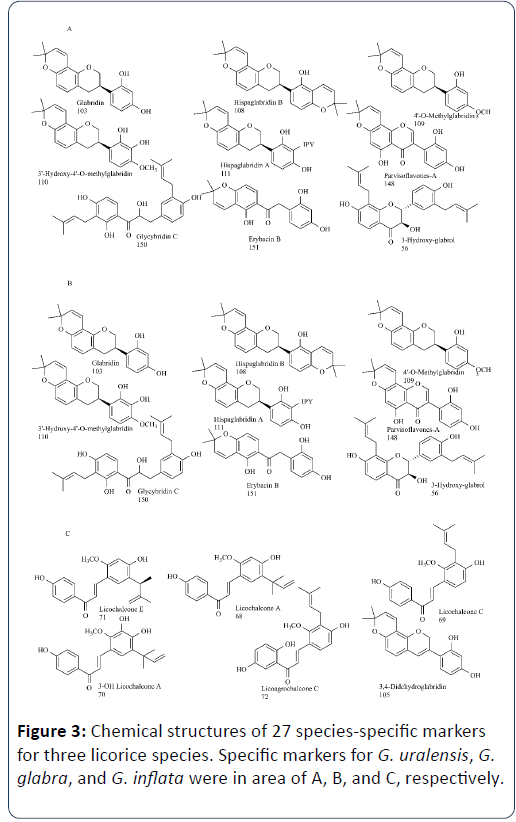
Many studies have confirmed that glabridin, licochalcon A and glycycoumarin were species-specific markers for G. glabra, G. inflata and G. uralensis respectively [10,32,39,45]. Although Kondo K, et al noted that there was no detection of the three species-specific constituents from 6% of licorices examined [39],they were still important markers for the species identification of licorice.
Some studies identified several novel markers among three licorice species. For example, Song W, et al. discovered a total of 27 species-specific markers (Figure 3), they met the requirements of high interspecies variance, relatively high contents, and nonoverlapped content ranges at 95% confidence interval [32].
Li G, et al. noted that only G. uralensis root preparations contained licoricidin (12) [45]. Farag MA, et al. reported that two aryl coumarins, glycyrin isomers (43 and 47) and an indolyl flavanone conjugate identified as licorice glycoside E (21) were identified in G. uralensis [10].
Rizzato G, et al. suggested that licochalcone A/C/E and licochalcone D were species-specific markers for G. inflata [40]. Farag MA, et al. noted that cadaverine, inflacoumarin A (32), glyinflanin A (51), licochalcone C (41) and D (35) were present exclusively in G. inflata extracts [10].
Rizzato G, et al. reported that in addition to kanzonol Y (22) and glabridin (25), which are prenylated flavonoids known to be markers for G. glabra species, 2,4,4′-trimethoxychalcone (36),guangsangon F (33), schaftoside (34) and hispaglabridin A (48) were detected only in G. glabra [40]. Farag MA, et al. noted that G. glabra showed two chemotaxonomic markers including the isoprenylated flavanone derivatives glabrol (50) and 3-hydroxy glabrol (45) [10]. Fang S, et al. noted that 3-hydroxyglabrol, gancaonin H and glyasperin N were present exclusively in G. glabra extracts [50].
Conclusions
In this review, we summarized the interspecific chemical variations in terms of the types and contents of triterpenoid saponins, flavonoids and polysaccharides and species-specific markers among three medicinal licorice species stipulated in Chinese pharmacopoeia. The chemical profiles of licorice vary significantly due to different plant species, which thus affect their quality and therapeutic effects. It is suggested that when used for given medicinal purposes, the licorice specie which was rich in target components could be superior to the other species for the corresponding bioactivities. Therefore, the species of licorice should be selected depending on these different properties when it is used for medicinal purposes.
In addition, because the content of chemical components of licorice is also affected by their geographic area, growth years, growth environment, harvesting and processing, we will further study their relationship to guarantee safe and effective use of licorice.
References
- Li YJ, Chen J, Li Y, Li Q, Zheng YF, et al. (2011) Screening and characterization of natural antioxidants in four Glycyrrhiza species by liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J Chromatogr A 1218: 8181-8191.
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, et al. (2008) Antiviral effects of Glycyrrhiza species. Phytother Res 22: 141-148.
- Qiao X, Liu CF, Ji S, Lin XH, Guo DA, et al. (2014) Simultaneous determination of five minor coumarins and flavonoids in Glycyrrhiza uralensis by solid-phase extraction and high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Planta Med 80: 237-242.
- Asl MN, Hosseinzadeh H (2008) Review of pharmacological effects of Glycyrrhiza sp. and its bioactive compounds. Phytother Res 22: 709-724.
- Lee HJ, Yoon MY, Kim JY, Kim Y, Park HR, et al. (2008) Antioxidant Activity of Glycyrrhiza uralensis Fisch Extracts on Hydrogen Peroxide-induced DNA Damage in Human Leucocytes and Cell Death in PC12 Cells. Food Sci Biotechnol 17: 343-348.
- Kim KR, Jeong CK, Park KK, Choi JH, Park JHY, et al. (2010) Anti-Inflammatory Effects of Licorice and Roasted Licorice Extracts on TPA-Induced Acute Inflammation and Collagen-Induced Arthritis in Mice. Biomed Res Int 2010: 709378.
- Tanaka A, Horiuchi M, Umano K, Shibamoto T (2008) Antioxidant and anti‐inflammatory activities of water distillate and its dichloromethane extract from licorice root (Glycyrrhiza uralensis) and chemical composition of dichloromethane extract. J Sci Food Agr 88: 1158-1165.
- Fiore C, Eisenhut M, Krausse R, Ragazzi E, Pellati D, et al. (2008) Antiviral effects of Glycyrrhiza species. Phytother Res 22: 141-148.
- Tao W, Duan J, Zhao R, Li X, Yan H, et al. (2013) Comparison of three officinal Chinese pharmacopoeia species of Glycyrrhiza based on separation and quantification of triterpene saponins and chemometrics analysis. Food Chem 141: 1681-1689.
- Farag MA, Andrea P, Wessjohann LA (2012) Comparative metabolite profiling and fingerprinting of medicinal licorice roots using a multiplex approach of GC-MS, LC-MS and 1D NMR techniques. Phytochemistry 76: 60-72.
- Fu Y, Chen J, Li YJ, Zheng YF, Li P (2013) Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem 141: 1063-1071.
- Zhou Y, Ho WS (2014) Combination of liquiritin, isoliquiritin and isoliquirigenin induce apoptotic cell death through upregulating p53 and p21 in the A549 non-small cell lung cancer cells. Oncol Rep 31: 298-304.
- Wang W, Hu X, Zhao Z, Liu P, Hu Y, et al. (2008) Antidepressant-like effects of liquiritin and isoliquiritin from Glycyrrhiza uralensis in the forced swimming test and tail suspension test in mice. Prog Neuro-psychoph 32: 1179-1184.
- Nakatani Y, Kobe A, Kuriya M, Hiroki Y, Yahagi T, et al. (2017) Neuroprotective effect of liquiritin as an antioxidant via an increase in glucose-6-phosphate dehydrogenase expression on B65 neuroblastoma cells. Eur J Pharmacol 815: 381-390.
- Kim JY, Park SJ, Yun KJ, Cho YW, Park HJ, et al. (2008) Isoliquiritigenin isolated from the roots of Glycyrrhiza uralensis inhibits LPS-induced iNOS and COX-2 expression via the attenuation of NF-kappaB in RAW 264.7 macrophages. Eur J Pharmacol 584: 175.
- Fu Y, Hsieh TJ, Kunicki J, Lee MY, Darzynkiewicz Z, et al. (2004) Licochalcone-A, a novel flavonoid isolated from licorice root (Glycyrrhiza glabra), causes G2 and late-G1 arrests in androgen-independent PC-3 prostate cancer cells. Biochem Bioph Res Co 322: 263-270.
- Chen M, Theander TG, Christensen SB, Hviid L, Zhai L, et al. (1994) Licochalcone A, a new antimalarial agent, inhibits in vitro growth of the human malaria parasite Plasmodium falciparum and protects mice from P. yoelii infection. Antimicrob Agents Ch 38: 1470-1475.
- Kwon HS, Park JH, Kim DH, Kim YH, Park JHY, et al. (2008) Licochalcone A isolated from licorice suppresses lipopolysaccharide-stimulated inflammatory reactions in RAW264.7 cells and endotoxin shock in mice. J Mol Med 86: 1287-1295.
- Kim JK, Shin EK, Park JH, Kim YH, Park JH (2010) Antitumor and antimetastatic effects of licochalcone A in mouse models. J Mol Med 88: 829-838.
- Kim HJ, Lim SS, Park IS, Ji SL, Ji YS, et al. (2012) Neuroprotective Effects of Dehydroglyasperin C through Activation of Heme Oxygenase-1 in Mouse Hippocampal Cells. J Agric Food Chem 71: 5583-5589.
- Cheng A, Wan F, Wang J, Jin Z, Xu X (2008) Macrophage immunomodulatory activity of polysaccharides isolated from Glycyrrhiza uralensis Fish. Int Immunopharmacol 8: 43-50.
- Zhang CH, Yu Y, Liang YZ, Chen XQ (2015) Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int J Biol Macromol 79: 681-686.
- Ayeka PA, Bian Y, Mwitari PG, Chu X, Zhang Y, et al. (2016) Immunomodulatory and anticancer potential of Gan cao (Glycyrrhiza uralensis Fisch.) polysaccharides by CT-26 colon carcinoma cell growth inhibition and cytokine IL-7 upregulation in vitro. Bmc Complem Altern M 16: 1-8.
- Chang YP, Bi WX, Yang GZ (1989) Studies on the anti-virus effect of Glycyrrhiza uralensis Fish. polysaccharide. China J Chin Mater Med 14: 236- 238.
- Zhu Z, Tao W, Li J, Guo S, Qian D, et al. (2016) Rapid determination of flavonoids in licorice and comparison of three licorice species. J Sep Sci 39: 473-482.
- Xie J, Zhang Y, Wang W, Hou J (2014) Identification and Simultaneous Determination of Glycyrrhizin, Formononetin, Glycyrrhetinic Acid, Liquiritin, Isoliquiritigenin, and Licochalcone A in Licorice by LC-MS/MS. Acta Chromatogr 26: 507-516.
- Kroes BH, Beukelman CJ, Aj VDB, Wolbink GJ, Van DH, et al. (1997) Inhibition of human complement by beta-glycyrrhetinic acid. Immunol 90: 115-120.
- Wei JH, Zheng YF, Li CY, Tang YP, Peng GP (2014) Bioactive constituents of oleanane-type triterpene saponins from the roots of Glycyrrhiza glabra. J Asian Nat Prod Res 16: 1044-1053.
- Plohmann B, Bader G, Hiller K, Franz G (1997) Immunomodulatory and antitumoral effects of triterpenoid saponins. Die Pharmazie 52: 953-957.
- Jr FR, Biglieri EG, Shackleton CH, Irony I, Gomezfontes R (1991) Licorice-induced hypermineralocorticoidism. New Engl J Med 325: 1223-1227.
- Zhang Q, Ye M (2009) Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A 1216: 1954-1969.
- Song W, Qiao X, Chen K, Wang Y, Ji S, et al. (2017) Biosynthesis-Based Quantitative Analysis of 151 Secondary Metabolites of Licorice to Differentiate Medicinal Glycyrrhiza Species and Their Hybrids. Anal Chem 89: 3146-3153.
- Seki H, Muranaka T (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23: 4112-4123.
- Rauchensteiner F, Matsumura Y, Yamamoto Y, Yamaji S, Tani T (2005) Analysis and comparison of Radix Glycyrrhizae (licorice) from Europe and China by capillary-zone electrophoresis (CZE). J Pharm Biomedl Anal 38: 594-600.
- TzuChien K, MingHuan S, GowChin Y (2009) Neuroprotective effects of glycyrrhizic acid and 18β-glycyrrhetinic acid in PC12 cells via modulation of the PI3K/Akt pathway. J Agric Food Chem 57: 754-761.
- Kalani K, Kushwaha V, Verma R, Murthy PK, Srivastava SK (2013) Glycyrrhetinic acid and its analogs: a new class of antifilarial agents. Bioorg Med Chem Lett 44: 2566-2570.
- TzuChien K, MingHuan S, GowChin Y (2010) Glycyrrhizic acid and 18β-glycyrrhetinic acid inhibit inflammation via PI3K/Akt/GSK3β signaling and glucocorticoid receptor activation. J Agric Food Chem 58: 8623-8629.
- Farag MA, Porzel A, Wessjohann LA (2015) Unequivocal glycyrrhizin isomer determination and comparative in vitro bioactivities of root extracts in four Glycyrrhiza species. J Advanc Res 6: 99-104.
- Kondo K, Shiba M, Nakamura R, Morota T, Shoyama Y (2007) Constituent properties of licorices derived from Glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol Pharm Bull 30: 1271-1277.
- Rizzato G, Scalabrin E, Radaelli M, Capodaglio G, Piccolo O (2017) A new exploration of licorice metabolome. Food Chem 221: 959-968.
- Montoro P, Maldini M, Russo M, Postorino S, Piacente S, et al. (2011) Metabolic profiling of roots of liquorice (Glycyrrhiza glabra) from different geographical areas by ESI/MS/MS and determination of major metabolites by LC-ESI/MS and LC-ESI/MS/MS. J Pharm Biomed Anal 54: 535-544.
- Xu R, Xiao Q, Cao Y, Yang J (2013) Comparison of the exposure of glycyrrhizin and its metabolites and the pseudoaldosteronism after intravenous administration of alpha- and beta-glycyrrhizin in rat. Drug Res 63: 620-624.
- Ying C, Bo Z, Jia Q, Hui Z, Pharmacy CO (2014) Simultaneous Determination of 18 α-glycyrrhizic Acid and 18 β-glycyrrhizic Acid in Glycyrrhiza uralensis by HPLC. China Pharmacy 25: 746-748.
- Yang R, Li W, Yuan B, Ma Y, Zhou S, et al. (2016) Simultaneous determination of 18 α-glycyrrhizic acid and 18 β-glycyrrhizic acid in three licorice samples from different origin by HPLC∗. Chin J Pharm Anal 36: 1065-1071.
- Li G, Nikolic D, van Breemen RB (2016) Identification and Chemical Standardization of Licorice Raw Materials and Dietary Supplements Using UHPLC-MS/MS. J Agric Food Chem 64: 8062-8070.
- Liao WC, Lin YH, Chang TM, Huang WY (2012) Identification of two licorice species, Glycyrrhiza uralensis and Glycyrrhiza glabra, based on separation and identification of their bioactive components. Food Chem 132: 2188-2193.
- Wei LI, Song XB, Sun CR, Xia Q (2013) Content determination of polysaccharides in Radix Glycyrrhizae from three different species. Tianjin J Tradit Chin Med 30: 47-49.
- Zhao L, Cheng ZM, Shu-Yong MU, Zhu JW, Pan HX (2005) Content of Glycyrrhizic Acid and Polysaccharide of Cultivated Glycyrrhiza Root. Arid Land Geogr 28: 843-848.
- Wei LI, Xia Q, Sun C, Song X, Pharmacy DO (2014) Analysis of Monosaccharide Compositions of Various Kinds of Liquorice Polysaccharide by HPLC Precolumn Derivatization. J Liaoning Univ Tradit Chin Med 16: 56-58.
- Fang S, Qu Q, Zheng Y, Zhong H, Shan C, et al. (2016) Structural characterization and identification of flavonoid aglycones in three Glycyrrhiza species by liquid chromatography with photodiode array detection and quadrupole time‐of‐flight mass spectrometry. J Sep Sci 39: 2068-2078.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences