
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 70
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Mimetic peptides based on promiscuous enzyme as asymmetric catalyst in Aldol and Michael reactions
Saadi Bayat
1,2
and
Basyaruddin A Rahman
1
1
Universiti Putra Malaysia, Malaysia
2
Tofigh Daru Research and Engineering Company, Iran
B
iocatalysis has emerged as an elegant and green tool for modern organic synthesis due to its high efficiency, good selectivity
and environmental acceptability. Although, an enzyme is capable of catalyzing a specific reaction effectively, some unexpected
experimental results have indicated that many enzymes are catalytically promiscuous. Mimetic peptides based on enzyme as a kind
of important chiral scaffold are broadly identified for their obvious advantages, diverse structures and ready accessibility. Based
on promiscuous aldo-keto-reductase enzymes, several mimetic peptides were designed which were synthesized and tested as
multifunctional organocatalysts in direct asymmetric aldol and Michael reactions. The asymmetric aldol and Michael reactions,
as the most prominent carbon-carbon bond formation reactions, are the central study issues in the field of asymmetric synthesis.
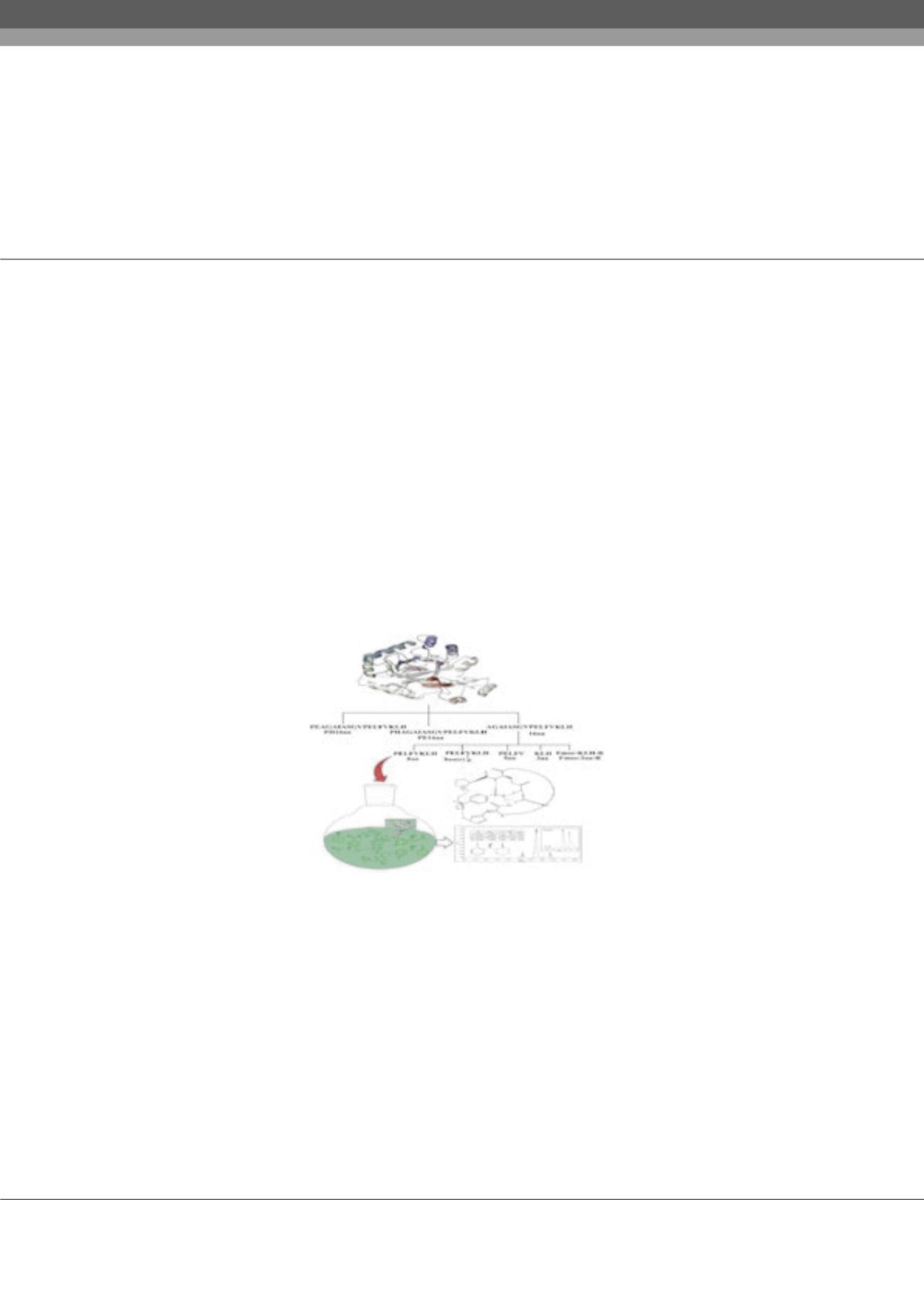
In this study, promiscuous aldo-ketoreductase (AKR) is used to catalyze aldol reaction between aromatic aldehydes and ketones.
Good yield (up to 75%), moderate enantioselectivity (60%), and high diastereoselectivity (dr) up to 93/7 (anti/syn) were obtained.
Several mimetic peptides from AKR’s active site were designed and synthesized as asymmetric catalysts in the aldol and Michael
reactions. The corresponding aldol products were produced with high yields (up to 97%) and excellent diastereoselectivities (up to
99/1) and enantioselectivities (up to 99.9) under mild reaction conditions. These peptides exhibit excellent catalytic activity in terms
of yield, diastereoselectivity and enantioselectivity. The secondary structures of peptide catalysts provide an understanding of their
mechanism.
Figure 1:
Synthesized mimetic peptides as asymmetric organocatalyst
Biography
Saadi Bayat received his BSc in Applied Chemistry at Buali Sina University (Hamedan, Iran, 2000). He completed his Postgraduation with MSc in Organic
Chemistry at Kharazmi University (Tehran, Iran, 2008). He had enrolled in PhD program at Department of Chemistry, Faculty of Science of University of Putra
Malaysia (UPM), under supervision of Prof. Dr. Mohd Basyaruddin Abdul Rahman. The following year, he was offered scholarship from Graduate Research
Assistance (GRA), UPM. Moreover, his research program focuses on mimetic peptide as asymmetric catalysis. He employed strategies that include organo-catalyst
design, and the application of these approaches to construct asymmetric C-C bond forming and esters hydrolysis. He was as a Postdoctoral Research Fellow for
a year (May 2014-June. 2015) in UPM. He has been selected to receive Endeavor scholarship from Australian Government and has joined Dr. Bellinda Abott in La
Trobe University, Melbourne (July 2015-January 2016).
saadibayat@yahoo.comSaadi Bayat et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-002
















