
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 67
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Efficient method for the synthesis of novel enantiomerically enriched derivatives of propargylglycine
Anna F Mkrtchyan
1,2
, Ashot S Saghyan
1,2
, Liana A Hayriyan
1,2
, Ani J Karapetyan
1,2
, Zorayr Z Mardiyan
1,2
, Hayarpi M Simonyan
1,2
and
Hovhannes Adonc
2
1
Scientific and Production Center “Armbiotechnology” - NAS, Armenia
2
Yerevan State University, Armenia
A
lkyne-containing amino acids are versatile structures readily available by a number of methods and are accessible using very
few transformations from economical starting materials. They can be functionalized by many chemical functions and offer a
wide range of possible transformations. Particularly, unsaturated α-amino acids give access to many synthetic applications in all
fields of chemistry. Among them, metal catalyzed cross-coupling reactions and cross metathesis are commonly used to generate
peptide modifications and cyclization. They are very interesting and useful tools for “Click” Chemistry in peptidomimetic drug
design or covalent modification of proteins. They can also be incorporated in compounds as beta-turn inducer to promote secondary
structures. Finally they can be used for the preparation of stapled peptides. Some such amino acids are commercially attainable in
enantiomerically pure form. Here, we present a stereoselective approach to synthesize unsaturated α-amino acids in optically active
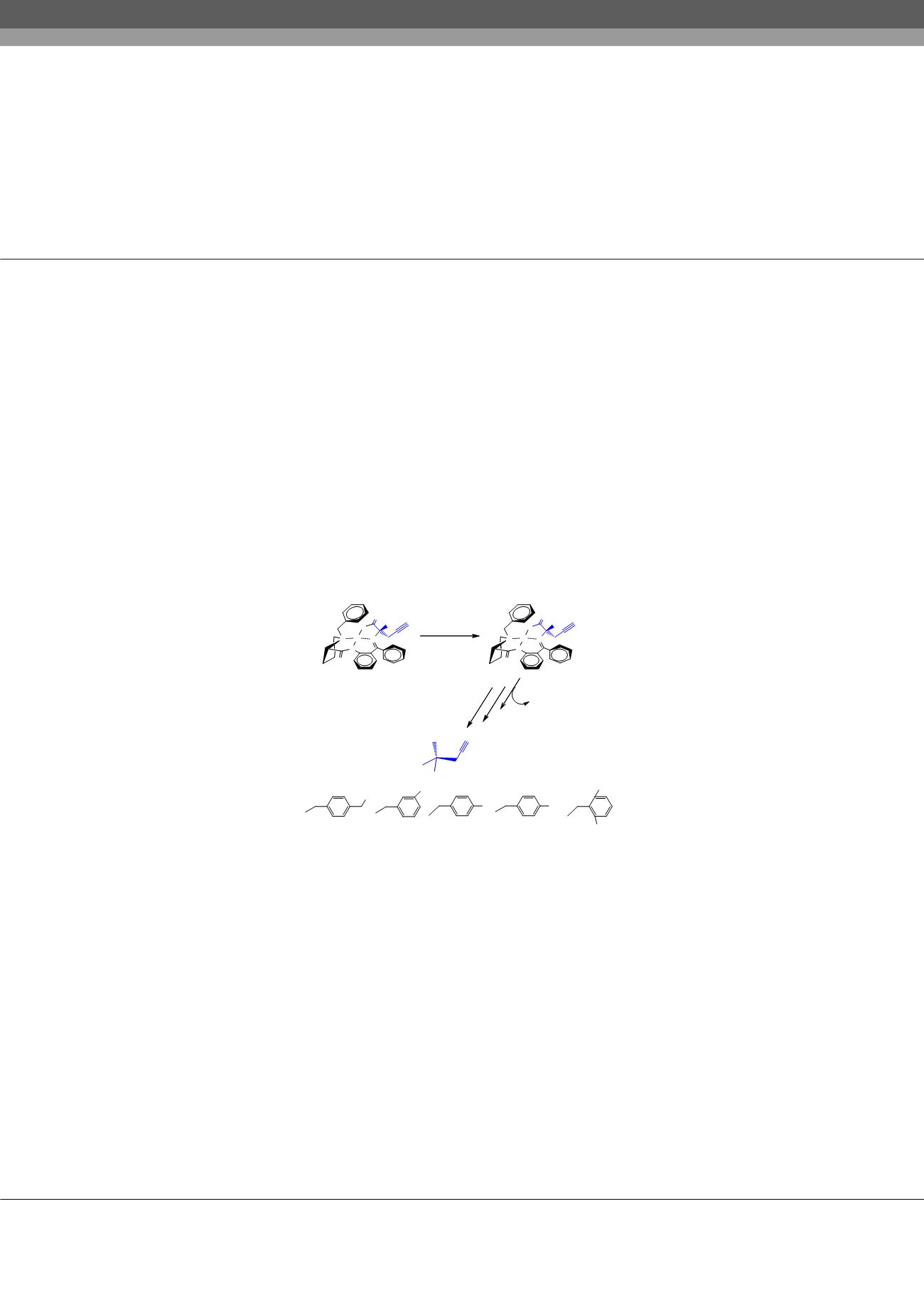
form. As a starting amino acid synthon for the asymmetric synthesis of amino acids NiII square-planar complexes of Schiff ’s bases
of propargylglycine with chiral auxiliary (S)-2-N-(N`-benzyl-prolyl)aminobenzophenone (BPB) (1) was taken. As a result effective
methods of asymmetric synthesis for novel enantiomerically enriched derivatives of (S)-propargylglycine (S)-propargylglycine (ee >
80%) was developed.
Biography
Anna F Mkrtchyan works in Institute of Pharmacy of Yerevan State University and SPC “Armbiotechnology” NAS RA. She got her PhD degree in Chemistry in 2013
specializing in Bioorganic Chemistry.
anna_mkrtchyan@ysu.amAnna F Mkrtchyan et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-002
N Ni
O
O
N
N
O
H
(S)
N Ni
O
O
N
N
O
R
R - Ha l
2( a - e )
DMF ,
Na OH
(S)
(S)
(S)
Ha l : Cl ,
Br
1
3( a - d)
Cl
Cl
( e )
Br
CF
3
CF
3
Br
( a )
(b)
( c )
(d)
(
S
)
- BPB
i
ii
iii
HOOC
(S)
H
2
N
R
2N HCl ,
50
o
C
( i ) ;
Dowex
50
( ii ) ;
cr ysta lli zati on
C
2
H
5
OH/H
2
O 1/1
( iii )
















