Reticulocyte Haemoglobin as a Routine Parameter in Preoperative Iron Deficiency Assessment
Christian Hönemann1*, Olaf Hagemann1, Dietrich Doll2, Markus M Luedi3, Marie-Luise Ruebsam4, and Patrick Meybohm5
1 Department of Anesthesia and Intensive Care Medicine, Marienhospital Vechta gGmbH, University of Münster, Germany
2 Department of General and Proctosurgery, Marien hospital Vechta gGmbH, Vechta, Germany
3 Department of Anaesthesiology and Pain Medicine, University of Bern, Bern,Switzerland
4 Department of Anesthesiology, St Marien hospital Vechta, Vechta, Germany
5 Director of the Clinic and Polyclinic for Anaesthesiology, University Hospital Würzburg, Würzburg, Germany
- *Corresponding Author:
- Christian Hönemann
Department of Anesthesia and Intensive Care Medicine,
Marienhospital Vechta gGmbH,
Marienstraße 6-8, 49377 Vechta
E-mail: christian.hoenemann@kh-vec.de
Received Date: December 22, 2020; Accepted Date: January 05, 2021 Published Date: January 12, 2021
Citation: Hönemann C, Hagemann O, Doll D, Luedi MM, Ruebsam ML, Meybohm P (2021) Reticulocyte Haemoglobin as a Routine Parameter In Preoperative Iron Deficiency Assessment. Endocrinol Metab Vol. 5 No.1:154.
Abstract
The measurement of haemoglobin content of reticulocytes (RET-He, reticulocyte haemoglobin equivalent) is an inexpensive parameter for the diagnosis and monitoring of iron supply to erythropoiesis. With the reticulocyte count giving a direct indication of the current quantity of erythropoiesis, the haemoglobin content of the reticulocytes measures iron supply to erythropoiesis and thus the quality of the cells. Changes in iron status of erythropoiesis can thus be detected in a much timelier fashion. In many hospitals, this method is already available, but underappreciated and used. A differentiated anemia diagnosis via other means, example, via ferritin, transferrin saturation, iron etc. necessarily increases laboratory costs. In the first pillar of Patient Blood Management, the preoperative anemia diagnosis, measuring RET-He could thus be a valuable option, since the costs of measurement are around 0.70€-1.00€. In contrast, costs are much higher for the traditional parameters ferritin (17.76€ as per German private healthcare system) and transferrin saturation (6.71€). More studies are urgently indicated to further analyze the role RET-He can play in Patient Blood Management.
Keywords
Iron deficiency; Anemia; Patient blood management; Reticulocyte haemoglobin equivalent
Introduction
More than 30% of the current worldwide population present with anemia with globally relevant medical and health economic repercussions [1]. As per the World Health Organization (WHO), anemia is described as a state in which the number of red blood cells is too low or the capability to transport oxygen is insufficient to match physiological demand [2]. Quantitatively, this amounts to a haemoglobin concentration (Hb)<13 g/dl (8 mmol/l) for men, Hb<12 g/dl (7.4 mmol/l) for women or <11 g/dl (6.8 mmol/l) for pregnant women indicating anemia [2]. With at a minimum 50% of cases, iron deficiency is the most frequent cause of anemia. Globally, iron deficiency is the most frequent form of malnutrition of humanity. An estimated two billion people worldwide suffer from iron deficiency. The prevalence in Europe is about 5%-10%, for women of childbearing age approximately 20%. Infants and toddlers are further at-risk populations [3]. At the same time, iron is a vital micronutrient for all living cells. As a key component of a host of enzymes, it holds a pivotal role in many different metabolic processes and is thus indispensable for human life. A lack of sufficient iron or a disturbance of its functional availability consequently has a significant impact on the whole body and is the pathological mechanism of many different classical clinical symptoms and indications from almost all medical disciplines. An especially pivotal role, however, is occupied by iron metabolism disorders in the field of haematology, as they necessarily impair the production of heme and thus precipitate, sooner or later, the development of anemia. Depending on the severity, three stages are described: Storage Iron Deficiency (ID), iron-deficient erythropoiesis (latent ID) and Iron Deficiency Anemia (IDA). A negative iron balance first leads to a storage iron deficiency (stage I). At this stage, while iron storage is being depleted, there is still sufficient iron to supply erythropoiesis. At the stage of iron deficient erythropoiesis (stage II), the supply to the erythropoietic precursors in the bone marrow is impaired, but haemoglobin is still within the reference interval. Once the haemoglobin-deficient population is large enough to depress the overall haemoglobin concentration below the reference interval, iron deficiency anemia has formed (stage III) [4].
The symptoms of anemia such as fatigue, shortness of breath, tachycardia and headaches are often mild, but have a significant impact on quality of life. A sustained insufficient oxygen supply due to an undiscovered or untreated anemia can have a significant impact on organ function. The early diagnosis of anemia at an early stage can allow a timely intervention and is thus of high importance. Anemia in and of itself is not a disease but a symptom that can be traced back to various other pathological conditions, which can lead to yet more severe symptoms of their own. Recognizing the issue early is thus crucial, especially preoperatively as part of patient blood management [1] and the concepts of “prehabilitation” [5] and “Enhanced Recovery after Surgery” (ERAS) [6]. Patient Blood Management is focused on a comprehensive anemia management, the minimization of (unnecessary) iatrogenic blood loss, and the reliance on natural anemia tolerance with a rational use of transfusions with red cell concentrates. To curb frailty syndrome, evidence in favour of a pre-habilitation for infirm patients is being verified, including the question as to whether the risks and results associated with surgery can be modulated by comprehensive geriatric care. For the ERAS concept, guidelines were developed for a screening of patients for a nutritional status indicating an added risk of adverse results due to malnutrition. In addition, methods for providing nutritional support and preoperative and postoperative optimization of the nutritional status have been developed.
Diagnosis of Iron Deficiency
The reference method for the assessment of iron supplies in the bone marrow and the diagnosis of iron deficiency is Prussian blue staining [7,8]. This method, however, requires a bone marrow aspiration, an invasive and painful intervention.
Alternatively, serum ferritin levels and Transferrin Saturation (TSAT) are considered good indicators for iron deficiency [7]. Traditional biochemical markers for the examination of iron status such as transferrin and ferritin are, however, confounded by, example, an inflammation over the cause of an acute phase reaction, malignancies, or chronical rheumatic problems to such a degree that an accurate clinical interpretation is hard to impossible. Thus, while low ferritin levels unambiguously indicate a lack of storage iron, normal or increased levels do not allow a clear conclusion. During chronic disease such as rheumatoid arthritis, but also liver disease, tumor, or terminal renal insufficiency (such as in patients under dialysis), ferritin can even be increased in the presence of iron deficiency [9,10]. TSAT, on the other hand, can be influenced by circadian variability of serum iron. The measurement of soluble transferrin receptor levels is part of a more elaborate workup of complicated cases of anemia. The sTfR is normally not determined alone but in conjunction with serum transferrin, serum ferritin and the reticulocyte count with reticulocyte haemoglobin. via sTfR, the current iron demand can be estimated, while ferritin mirrors the iron available in storage. Both values together give a more comprehensive view of the iron status that can be quantified through the sTfR-Ferritin- Index (sTfR concentration/log ferritin concentration).
Reticulocyte Haemoglobin RET-He
Due to the 120-day lifespan of red blood cells, iron deficits or changes in iron status of erythropoiesis are only recognizable at a very late stage when using classic haematological parameters such as Haemoglobin (HB), Mean Corpuscular Volume (MCV), Mean Cellular Haemoglobin (MCH) or even with the percentage of hypochromic red blood cells (%Hypo). The haemoglobin content of reticulocytes, RET-He (reticulocyte haemoglobin equivalent), is a parameter for the diagnosis and monitoring of iron supply in erythropoiesis. This parameter is correctly diagnosing iron deficiency during the acute phase of an inflammation response. Reticulocytes are the precursors of mature erythrocytes, are flushed from the bone marrow into the peripheral blood and mature there over the course of two days to a fully mature red blood cell. Determining the number or concentration of reticulocytes thus provides timely information on the “quantity” of erythropoiesis in the bone marrow. On the other hand, determining the haemoglobin content of reticulocytes mirrors the iron supply to erythropoiesis and thus gives insight into the “quality” of erythropoiesis. Changes in the iron status of erythropoiesis can this be recognized far earlier than looking at the haemoglobin content of mature red cells, the MCH.
Measurement Technology of Reticulocyte Haemoglobin RET-He
The measurement of RET-He is provided in less than a minute as part of a complete blood count (from EDTA monovette) on a haematology analyser using fluorescence flow cytometry [11].
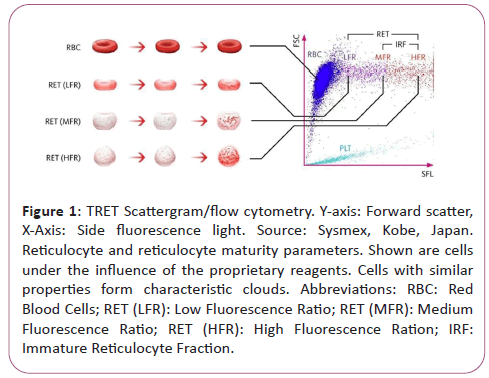
During flow cytometry, cells and particles are characterized while passing a small flow chamber. First, the blood sample is aspirated and split to then be diluted in a pre-set ratio and stained with a proprietary fluorescence stain specifically binding to nucleic acids. Subsequently, the sample is transported to the flow chamber and irradiated with the light of a semiconductor laser beam. This results in three emitted signals the characterization of which allows the distinction of various cell types:
• Forwards Scatter Light (FSL),
• Side Scatter Light (SSL),
• Side Fluorescence Light (SFL).
The intensity of forward scatter allows conclusions as to the cell volume, the side scatter light as to the cell components such as nucleus and granula, and the side fluorescence indicates the amount of nucleic acid content (DNA and RNA) in the cell. Increased nucleic acid content corresponds to a stronger fluorescence signal. Cells with similar properties form characteristic clouds in a special scatter plot diagram (scatter gram).
Scatter gram of the XN-Series RET Channel (Figure 1).
Figure 1: TRET Scattergram/flow cytometry. Y-axis: Forward scatter, X-Axis: Side fluorescence light. Source: Sysmex, Kobe, Japan. Reticulocyte and reticulocyte maturity parameters. Shown are cells under the influence of the proprietary reagents. Cells with similar properties form characteristic clouds. Abbreviations: RBC: Red Blood Cells; RET (LFR): Low Fluorescence Ratio; RET (MFR): Medium Fluorescence Ratio; RET (HFR): High Fluorescence Ration; IRF: Immature Reticulocyte Fraction.
Recognition of a Latent Iron Deficiency via Reticulocyte Hemoglobin
Latent and subclinical Iron Deficiency (ID) describes clinical conditions with iron deficiency in the absence of anemia. Without supplementing iron, however, these conditions in all regularity lead to a full-fledged Iron Deficiency Anemia (IDA) forming within weeks or months and thus are among the most frequent causes of anemia. Especially for pregnant women, ID is a key issue, as the development of IDA is associated to a high degree with complications for both mothers and neonates.
The incidence of ID for infants and toddlers is likewise high, and frequently leads to a clinical manifestation as IDA. Consequently, especially preoperatively, recognition of a latent and subclinical ID is necessary to induce changes in nutrition and supplemental medication which can prevent development of IDA [12].
In this context, the measurement of reticulocyte haemoglobin to predict iron deficiency has proven a valuable option and has been tested in several different patient cohorts. In a study on 501 blood donors, it was shown that RET-He has an ability comparable to the soluble Transferrin Receptor (sTfR) to identify latent ID. The parameters sTfR and Ret-He indicated a latent iron deficiency in 148 and 135 donors, respectively. RET-He showed, in comparison with sTfR, a sensitivity of 92.7% a specificity of 97.16%, a PPV of 93.1% and an NV of 96.3%. Serum ferritin, TIBC and serum iron each had a comparatively lower sensitivity of 87.16%, 79.7% and 77.7% [13].
In premenopausal and pregnant women, RET-He also showed significant differences between ID and non-ID subjects and proved useful in the diagnosis of a subclinical ID [7,8]. In contrast to biochemical iron markers, which are more expensive and less accurate, the use of RET-He is an inexpensive and fast screening tool for the assessment of a subclinical or latent ID in various patient cohorts.
Mehta et al. verified the ability of RET-He and serum ferritin to predict iron in the bone marrow during IDA in an adult patient cohort. Their results show that there is a high correlation between RET He and serum ferritin (r=0.786, P<0.0001) and an almost identical predictive value for bone marrow iron storage with an AUC of 0.89 for both [10].
In a study by Butarello et al. the analytical performance and diagnostic efficiency of the two parameters HYPO-He (percentage of hypochromic red blood cells with Hb<17 pg) and RET-He to identify iron deficient patients with (IDA) and without (ID) anemia was evaluated. With a sensitivity of 93.1% and a specificity of 95.1% at a cut-off of 30.6 pg, RET-He showed a remarkable ability to identify IDA and scored better than HYPO-He at a cutoff of 0.9%, which achieved 84.5% and 95.7% for sensitivity and specificity [9]. Butarello et al. see an important role in the differential diagnosis of anemia for erythrocyte indices. These indices are calculated from various hematological parameters like the RBC count, hemoglobin concentration and hematocrit. Taken together, these studies suggest that a combination of erythrocyte indices and reticulocyte parameters especially RET-He unlock promising opportunities for IDA recognition and assessment of iron stores in the bone marrow. They are not affected by acute phase [9,14,15] and display a much lower biological variability than TSAT and ferritin [16].
Differentiating IDA from Anemia of Chronic Disease (ACD)
Anemia of Chronic Disease (ACD) is the second most common form of anemia worldwide [17]. In most cases, ACD develops as a normocytic anemia, i.e., MCV is not being affected. Nonetheless, the distinction between IDA, ACD and a combination ACD/IDA is difficult. With RET-He being a useful parameter for the detection of ID and IDA in various patient cohorts, it can also support the distinction between IDA and ACD. A study by Canals et al. first reported significant differences between RET-He levels in IDA and ACD patients [11]. Later, the capability of RET-He to distinguish IDA and ACD was demonstrated by Urrechaga et al. Notably, not just RET-He but also HYPO-HE showed significant differences between patients with iron deficiency, chronic kidney disease and patients on haemodialysis. The results of the IDA group reflected a state of iron deficiency (low ferritin), low iron availability (low MCH, high percentage of hypochromic red blood cells (HYPE-He) and iron-restricted erythropoiesis (low RET-He). For patients on haemodialysis, the reticulocyte percentage demonstrated an increased erythropoietic activity, which due to treatment and good iron availability could be sustained (RET-He>30 pg). The results of ROC analysis for the diagnosis of iron deficiency were as follows: Ret-He: AUC 0.935, cut-off 29.8 pg, sensitivity 90.7%, specificity 83.1%. %Hypo-He: AUC 0.925, cut-off 3.5%, sensitivity 87.3%, specificity 88.0% [18].
Aside from RET-He, Delta-He has also come to be regarded as a useful parameter in the treatment of anemia of chronic disease. Delta-He is the difference between the haemoglobin content of mature erythrocytes and that of reticulocytes and thus reflects the trend of iron incorporation in erythrocytic precursors. It also provides information on the functional availability of iron and can be associated with inflammatory processes [19,20]. It was demonstrated that Delta-He differs significantly in patients with healthy subjects and ACD/IDA patients. Likewise, RET-He differs between control subjects and patients with ACD/IDA or IDA [21].
Discussion
For a rational and successful preoperative anemia diagnosis and therapy, a timely identification/screening of patients is crucial. For patients displaying iron deficiency with or without anemia, this allows an effective iron substitution in the context of Patient Blood Management.
The measurement of hemoglobin content of reticulocytes (RET-He, reticulocyte hemoglobin equivalent) is an inexpensive parameter for the diagnosis and monitoring of iron supply to erythropoiesis. With the reticulocyte count giving a direct indication of the current “quantity” of erythropoiesis, the hemoglobin content of the reticulocytes measures iron supply to erythropoiesis and thus the “quality” of the cells. Changes in iron status of erythropoiesis can thus be detected in a much timelier fashion.
In many hospitals, this method is already available, but underappreciated and -used. A differentiated anemia diagnosis via other means, e.g., via ferritin, transferrin saturation, iron etc. necessarily increases laboratory costs. In the first pillar of Patient Blood Management, the preoperative anemia diagnosis, measuring RET-He could thus be a valuable option, since the costs of measurement are around 0.70€-1.00€. In contrast, costs are much higher for the traditional parameters ferritin (17.76€ as per German private healthcare system) and transferrin saturation (6.71€). More studies are urgently indicated to further analyze the role RET-He can play in Patient Blood Management.
Conclusion
We recommend the evaluation of RET-He as a routine preoperative parameter to identify patients at risk for latent anemia in an inexpensive fashion in order to proactively initiate treatment in the spirit of prehabilitation and ERAS concepts, avoid complications and prevent extended hospitalization.
References
- Meybohm P, Richards T, Isbister J, Hofmann A, Shander A, et al. (2017) Patient blood management Bundles to facilitate implementation. Anasth Intensivmed 58: 16-29.
- World Health Organization (2011) Haemoglobin concentrations for the diagnosis of anemia and assessment of severity. World Health Organization.
- McLean E, Cogswell M, Egli I, Wojdyla D, De Benoist B (2009) Worldwide prevalence of anemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public Health Nutr 12: 444-454.
- Onkopedia Guideline: Recommendation for the diagnosis and therapy of hematological and oncological diseases, iron deficiency and iron deficiency anemia (German).
- Norris CM, Close JC (2019) Prehabilitation for the frailty syndrome: Improving outcomes for our most vulnerable patients. Anesth Analg 130: 1524-1533.
- Hedrick TL, McEvoy MD, Mythen MM, Bergamaschi R, Gupta R, et al. (2018) American Society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative gastrointestinal dysfunction within an enhanced recovery pathway for elective colorectal surgery. Anesth Analg. 126: 1896-1907.
- Levy S, Schapkaitz E (2018) The clinical utility of new reticulocyte and erythrocyte parameters on the Sysmex XN 9000 for iron deficiency in pregnant patients. Int J Lab Hematol 40: 683-690.
- Urrechaga E, Borque L, Escanero JF (2016) Clinical value of hypochromia markers in the detection of latent iron deficiency in nonanemic premenopausal women. J Clin Lab Anal 30: 623-627.
- Buttarello M, Pajola R, Novello E, Mezzapelle G, Plebani M (2016) Evaluation of the hypochromic erythrocyte and reticulocyte hemoglobin content provided by the Sysmex XE-5000 analyzer in diagnosis of iron deficiency erythropoiesis. Clin Chem Lab Med 54: 1939-1945.
- Mehta S, Goyal LK, Kaushik D, Gulati S, Sharma N, et al. (2016) Reticulocyte hemoglobin vis-a-vis serum ferritin as a marker of bone marrow iron store in iron deficiency anemia. J Assoc Physicians India 64: 38-42.
- Canals C, Remacha AF, Sarda MP, Piazuelo JM, Royo MT, et al. (2005) Clinical utility of the new Sysmex XE 2100 parameter - reticulocyte hemoglobin equivalent-in the diagnosis of anemia. Haematologica 90: 1133-1134.
- Ullrich C, Wu A, Armsby C, Rieber S, Wingerter S, et al. (2005) Screening healthy infants for iron deficiency using reticulocyte hemoglobin content. JAMA 294: 924-930.
- Tiwari AK, Bhardwaj G, Arora D, Aggarwal G, Pabbi S, et al. (2018) Applying newer parameter RET-He (reticulocyte haemoglobin equivalent) to assess latent iron deficiency (LID) in blood donors-study at a tertiary care hospital in India. Vox Sang 113: 639-646.
- Thomas L, Franck S, Messinger M, Linssen J, Thomé M, et al. (2005) Reticulocyte hemoglobin measurement - Comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin Chem Lab Med 43: 1193-1202.
- Thomas C, Thomas L (2002) Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem 48: 1066-1076.
- Van Wyck DB, Alcorn Jr H, Gupta R (2010) Analytical and biological variation in measures of anemia and iron status in patients treated with maintenance hemodialysis. Am J Kidney Dis 56: 540-546.
- Chaturvedi S, Moliterno AR, Merrill SA, Braunstein EM, Yuan X, et al. (2018) Chronic kidney disease, hypertension and cardiovascular sequelae during long term follow up of adults with atypical hemolytic uremic syndrome. Blood. 132: 3754.
- Urrechaga E, Borque L, Escanero JF. (2013) Erythrocyte and reticulocyte indices in the assessment of erythropoiesis activity and iron availability. Int J Lab Hematol 35: 144-149.
- Danielson K, Beshara S, Qureshi AR, Heimbürger O, Lindholm B, et al. (2014) Delta-He: A novel marker of inflammation predicting mortality and ESA response in peritoneal dialysis patients. Clin Kidney J 7: 275-281.
- De Mast Q, Van Dongen‐Lases EC, Swinkels DW, Nieman AE, Roestenberg M, et al. (2009) Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol 145: 657-664.
- De Mast Q, Van Dongen‐Lases EC, Swinkels DW, Nieman AE, Roestenberg M, et al. (2009) Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol 145: 657-664.

Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences