Catastrophic Antiphospholipid Antibody Syndrome (CAPS): A Catastrophe that Spares Rare!
Ankita Sheoran*
Department of Medicine, Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS), New Delhi, India
- *Corresponding Author:
- Ankita Sheoran
Department of Medicine,
Atal Bihari Vajpayee Institute of Medical Sciences (ABVIMS),
New Delhi,
India,
Tel: 08572828558;
E-mail: anki.sheoran94@gmail.com
Received date: January 02, 2023, Manuscript No. IPJCMT-22-15578; Editor assigned date: January 05, 2023, PreQC No. IPJCMT-22-15578 (PQ); Reviewed date: January 20, 2023, QC No. IPJCMT-22-15578; Revised date: March 23, 2023, Manuscript No. IPJCMT-22-15578 (R); Published date: March 31, 2023, DOI: 10.36648/IPJCMT.8.1.001
Citation: Sheoran A (2023) Catastrophic Antiphospholipid Antibody Syndrome (CAPS): A Catastrophe that Spares Rare! J Clin Med Ther Vol:8 No:1
Abstract
Catastrophic Antiphospholipid Antibody Syndrome (CAPS) sometimes represents the first manifestation of APS. We present case of a 30 years Indian male who presented with pulmonary renal involvement with thrombotic microangiopathy secondary to scrub typhus infection. A high index of suspicion for CAPS is important as early treatment can be life-saving.
Keywords
Catastrophic Antiphospholipid Syndrome (CAPS); Scrub typhus; Tropical infections; Thrombocytopenia; Leucocytosis
Introduction
Catastrophic APS (CAPS), a severe form of antiphospholipid syndrome, is characterized by accelerated and widespread small and medium vessel thromboses with multiple organ systems’ involvement and mortality approaching 50% despite aggressive multimodal intensive treatment [1]. A high suspicion for CAPS is important as early treatment can be life-saving. Diagnosis can be challenging as it shares clinical features with other life threatening conditions such as sepsis, disseminated intravascular coagulation, heparin induced thrombocytopenia, thrombotic microangiopathies (including TTP/HUS/HELLP). This case highlights the precedence of prompt diagnosis. Diagnosis of such rare diseases poses clinical challenges, and management is even more formidable, with gruesome fallouts.
Case Presentation
A 30 years Indian man presented with cough and hemoptysis, reduced urine output and generalized puffiness for 3 days, and breathlessness for 2 days. There was no associated fever, sore throat or any recent travel history [2]. At presentation, blood pressure was 200/120 millimeters of mercury, oxygen saturation was 85% on room air, had diffuse coarse crepitations all over on chest examination.
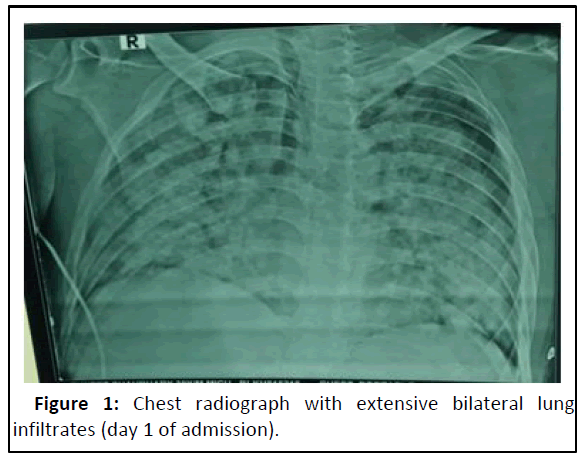
Blood investigations showed anemia (haemoglobin 7.8 gm/dL), neutrophilic leucocytosis (17000 cells/cumm), thrombocytopenia (80,000 per cumm) and deranged renal functions (urea/ creatinine 121/7.7 mg/dL). Arterial blood gas analysis showed hypoxic respiratory failure with PO2/FiO2 of 102. Chest radiograph showed bilateral lung infiltrates (Figure 1). HRCT chest showed multiple sub-centimetric lymph nodes largest being right paratracheal lymph node diffuse ground glass opacities with crazy paving pattern, suggesting diffuse alveolar hemorrhage. Urine was positive for proteinuria and hematuria. C-reactive protein and erythrocyte sedimentation rate were high (27.8 mg/dL and 109 mm for first hour, respectively) [3]. Procalcitonin and COVID RTPCR were negative. Blood culture and urine culture were sterile. Sputum for acid fast bacillus, fungal elements and aerobic growth was non-contributory. Antinuclear antibodies, anti-myeloperoxidase, anti-PR3, anti-GBM and complement factor C3/C4 levels were all normal.
Kidney biopsy was done in view of deranged renal functions and active urinary sediment. The biopsy showed ischemic wrinkling of capillary loops with few glomeruli showing fibrillary appearance, diffuse tubular injury with mild inflammation, dominant thrombotic microangiopathy changes in form of endothelial swelling, fragmentation of RBC’s, muco-intimal oedema and hyperplastic arteriopathy [4]. Immunohistochemistry was negative for IgM/IgG/IgG/C3/C1Q deposits.
Apart from broad spectrum antimicrobials and other supportive treatment, hemodialysis was performed in view of progressive derangement of renal function tests and anuria. However, hemoptysis and breathlessness worsened temporally, and bronchoscopy was done. Bronchial lavage was clear with few mucus particles [5]. Intra-procedure, his oxygen saturation level dropped to 30%, calling for abandonment of the procedure. He was electively intubated and put on mechanical ventilatory support with FiO2 100%.
In view of persistently raised serum fibrinogen levels and activated Partial Thrombin Time (aPTT), negative procalcitonin and cultures, possibilities of disseminated intravascular coagulation and thrombotic microangiopathy were considered. ADAMTS13 factor and von Willibrand factor levels and stool for shiga toxin were sent. Amongst other relevant investigations sent, lupus anticoagulant, beta-2 glycoprotein IgM, cardiolipin antibodies IgM were distinctively positive. To delineate the possible etiology, a host of tropical infections including leptospira, scrub typhus, malaria, dengue, tuberculosis, human immunodeficiency virus, serum galactomannan and beta-Dglucan were investigated, and found all negative except IgM positivity for scrub typhus.
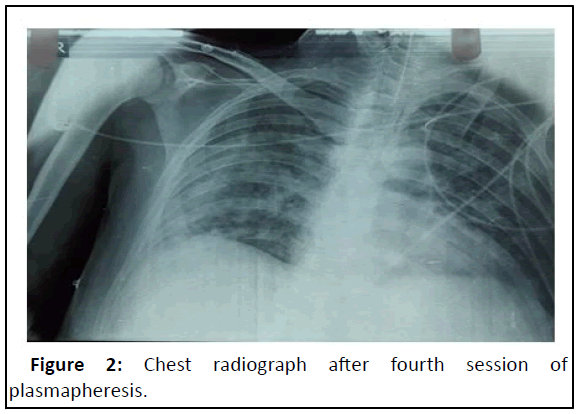
Diagnosis was revised as to ‘scrub typhus with catastrophic APLA with pulmonary renal syndrome and ARDS patient was started on anticoagulation with unfractionated heparin, intravenous methylprednisolone 1 gram daily and intravenous Rituximab 1000 milligrams (day 0 and 15 regimen). To our dismay, his condition didn’t improve much after 3 days of treatment [6]. Plasmapheresis was planned in view of failed pulse steroid treatment, and, after three sessions of plasmapheresis, he showed tangible improvement, with decrease in FiO2 requirement from 100% to 45%. Chest radiograph showed marked improvement (Figure 2). After being stable for 3 days on minimal ventilatory support settings, he was planned for weaning off the ventilator but unfortunately developed new onset of fever and worsened oxygenation parameters. Next day, he went into shock. Repeat chest radiograph showed right lower and middle zone consolidation with patchy opacities in left lung. Total leucocyte count was raised, and procalcitonin was positive. ET culture was positive for MDR klebsiella pneumoniae, sensitive to tigecycline and colistin. He was started on dual inotropic support and escalated antibiotics in view of klebsiella sepsis and septic shock. However, he showed no response to treatment, had refractory shock and succumbed to sepsis after 3 days.
(ADAMTS13, von Willibrand factor and Shiga toxin report received post-obituary, were negative).
Results and Discussion
Catastrophic APLA (CAPS), occasionally representing the first manifestation of APS. The classification criteria describe disease spectrum as definite CAPS, probable CAPS, CAPS like disease, microangiopathic APLA, and thrombotic storm. The term ‘microangiopathic APS’ has been proposed to encompass aPLpositive patients with predominant thrombotic microangiopathy features. Most cases in previously diagnosed APS patients are triggered by factors including infection, inadequate anticoagulation, and coexistence of systemic lupus erythematosus flare.
Life threatening organ involvement in CAPS includes diffuse alveolar hemorrhage, renal microvascular thrombosis, cardia and cerebral involvement. In our case, the initial presentation was of a pulmonary renal syndrome. The proposed mechanisms of pulmonary involvement include immune mediated injury to the lung’s microcirculation, pulmonary capillaritis, disruption of alveolar capillary basement membrane, microthrombosis, and mTOR Kinase induced endothelial proliferation causing vasculopathy [7]. CAPS nephropathy is attributed to thromboses in renal arteries or veins, intraparenchymal arteries and glomerular capillaries; histologically, marked by thrombotic microangiopathy. Other TMAs such as malignant hypertension, Thrombotic Thrombocytopenic Purpura (TTP), Hemolytic Uremic Syndrome (HUS), HELLP syndrome, and Heparin Induced Thrombocytopenia (HIT) may have similar presentations.
CAPS treatment is pillared on two aspects, one being therapeutic anticoagulation which is critical for treating the thrombotic events, and the additional therapies including plasmapheresis, Intravenous Immunoglobulin (IVIG), and/or immunomodulatory agents to suppress the cytokine cascade. The popular treatment approach is the ‘triple therapy,’ that is, anticoagulants, corticosteroids, plasma exchange, and/or IVIG. Other therapeutic modalities such as cyclophosphamide, rituximab and eculizumab may have a role as a second and/or third line therapy in the treatment of complicated, refractory/ relapsing CAPS patients.
Conclusion
An uncommon disease with a host of unusual and deadly presentations and multisystem involvement, a rheumatological emergency as CAPS mandates early intervention, both diagnostic as well as therapeutic. An assailing treatment approach can possibly halt the fulminant course of the disease.
Conflict of Interest
None.
References
- Cervera R, Rodriguez-Pinto I, Colafrancesco S, Conti F, Valesini G, et al. (2014) 14th Internationational congress on antiphospholipid antibodies task force report on catastrophic antiphospholipid syndrome. Autoimmun Rev 13:699–707
[Crossref] [Google Scholar] [PubMed]
- Asherson RA, Cervera R, de Groot P, Erkan D, Boffa MC, et al. (2003) Catastrophic Antiphospholipid Syndrome (CAPS): International consensus statement on classification criteria and treatment guidelines. Lupus 12:530–534
[Crossref] [Google Scholar] [PubMed]
- Garcia-Carrasco M, Mendoza-Pinto C, Macias-Diaz S, Vazquez de Lara F, Etchegaray-Morales I, et al. (2015) The role of infectious diseases in the catastrophic antiphospholipid syndrome. Autoimmun Rev 14:1066–1071
[Crossref] [Google Scholar] [PubMed]
- Deane KD, West SG (2005) Antiphospholipid antibodies as a cause of pulmonary capillaritis and diffuse alveolar hemorrhage: A case series and literature review. Semin Arthritis Rheum 35:154-165
[Crossref] [Google Scholar] [PubMed]
- de Azevedo FVA, Maia DG, de Carvalho JF, Rodrigues CEM (2018) Renal involvement in antiphospholipid syndrome. Rheumatol Int 38:1777-1789
[Crossref] [Google Scholar] [PubMed]
- Uthman I, Noureldine MHA, Ruiz-Irastorza G, Khamashta M (2019) Management of antiphospholipid syndrome. Ann Rheum Dis 78:155-161
[Crossref] [Google Scholar] [PubMed]
- Carmi O, Berla M, Shoenfeld Y, Levy Y (2017) Diagnosis and management of catastrophic antiphospholipid syndrome. Expert Rev Hematol 10:365-374
[Crossref] [Google Scholar] [PubMed]
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences