The Elusive 'Gut-Brain Axis' - New Directions in the Development of Metabolic Syndrome and Inflammation
Sadig R1*, Aramideh J2 and Wegrecki K1
1Faculty of Medicine, University of Notre Dame, Sydney, Australia
2The Brain and Mind Centre, Sydney Medical School, University of Sydney, Australia
- *Corresponding Author:
- Richard Sadig
Faculty of Medicine, University of Notre Dame
Sydney, 160 Oxford St
Darlinghurst NSW 2010, Australia
Tel: +61-2-95945671
Fax: +61-2-95333712
E-mail: richard.sadig1@my.nd.edu.au
Received Date: August 01, 2017; Accepted Date: August 05, 2017; Published Date: August 08, 2017
Citation: Sadig R, Aramideh J, Wegrecki K (2017) The Elusive 'Gut-Brain Axis' - New Directions in the Development of Metabolic Syndrome and Inflammation. Rep Endocr Disord Vol.1 No.1:1
The current paradigm of thinking about obesity and diabetes is primarily one of lifestyle - a bad diet, poor exercise, and in some cases, a predisposition. However, the link between the gut environment and the brain is only now being fully unravelled and more fully appreciated. The contribution of the effects of insulin on the central nervous system and the gut microbiome's effect on the brain. Insulin has been purported to act centrally on the brain to reduce food intake and body weight as well as regulate satiety and even cognition. In addition to insulin's effect on the brain is the varying levels of Bacteroidetes and Firmicutes in a host organism which is purported to dictate in part the efficiency of energy extraction of a given food source and the release of lipopolysaccharide (LPS) in response to a high-fat food source.
In this Editorial issue of Reports in Endocrine Disorders, we would like to report on some of the developments occurring in the discipline of endocrinology in terms of the way obesity and diabetes are viewed in lieu of emerging experiments.
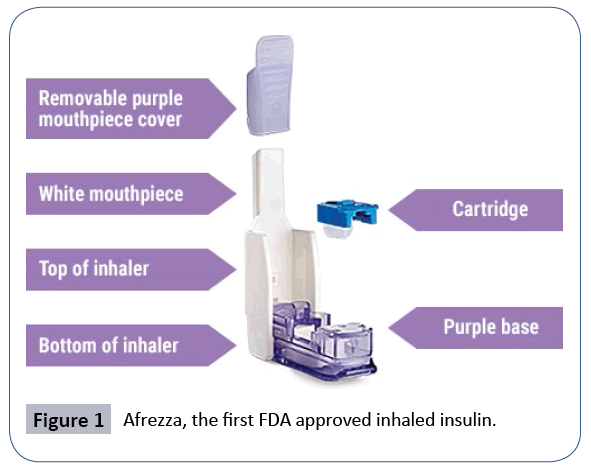
The first area of interest involves insulin's potentially centrally acting effect on the brain, described by Gray et al as an ability to impact "feeding behaviour and body energy stores, the metabolism of glucose and fats in the liver and adipose and various aspects of memory and cognition". This was shown in baboons who were given chronic administrations of intracerebroventricular (ICV) insulin which demonstrated decreases in baboon food intake and body weight [1]. The implication of this and other experiments is that peripheral insulin resistance may be translatable into a central nervous system resistance which may perpetuate a cascading problem. A deficiency or resistance in central insulin binding may result in overeating, lack of satiety, and weight issues in theory. Whilst still unknown, various attempts have been postulated to describe exactly how insulin reaches the brain - including via brain interstitial fluid (ISF), brain endothelial uptake or even local production of insulin in the brain [1]. Clinically, this has led to a big push for "intranasal insulin administration" as a way to regulate obesity, diabetes and even Alzheimer's disease. Afrezza became the first inhaled FDA approved insulin regimen in 2014 for the replacement of short acting insulin before meals in the management of diabetes (Figure 1) [2].
The second area of interest involves the so called 'obese' versus 'lean' microbiome subtypes - in mice - which postulate that a certain distribution of Bacteroidetes and Firmicutes in the gut may contribute to how efficiently a host organism may extract energy from a food source. Obese hosts are likely to be highly efficient at extracting energy from a food source whilst a lean metagenomic type is likely to be very inefficient at extracting energy from a food item [3]. Other studies in mice showed that germ-free high fat fed mice demonstrated lower comparative plasma lipopolysaccharide (LPS) levels than conventionally high fat fed-mice. LPS, amongst other signalling chemicals, are thought to be vital in contributing to the metabolic endotoxemia, low grade inflammation and other predecessors involved metabolic syndrome, insulin resistance and obesity [4].
Whilst this area of research is comparatively still in its infancy, we postulate that in the future, Faecal Microbiota Transplantation (FMT) post gut decontamination with an antibiotic may be a viable option in restoring or replacing obese microbiota types with lean microbiota types in the hope that it will reduce systemic endotoxemia and chronic inflammation, as well as to reduce obesity and the leptin associated pathways (Figure 2).
References
- Gray SM, Meijer RI, Barrett EJ (2014) Insulin Regulates Brain Function but How Does It Get There? Diabetes 63: 3992-3997.
- McGill JB, Ahn D, Edelman SV, Kilpatrick CR, Santos Cavaiola T (2016) Making Insulin Accessible: Does Inhaled Insulin Fill an Unmet Need? Advances in Therapy 33: 1267-1278.
- Turnbaugh PJ, Peter JT, Ruth EL, Michael AM, Vincent M (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-1131.
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM,et al. (2008) Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet-Induced Obesity and Diabetes in Mice. Diabetes 57: 1470.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences