ISSN : 2576-3911
Integrative Journal of Global Health
Soluplus as a Potential Enhancer of Cefixime Biopharmaceutical Properties through Solid Dispersion Prepared by Different Pharmaceutical Interventions
Poonam Mogal1* and Deeliprao Derle2
1Maratha Vidya Prasarak Samaj's College of Pharmacy, Nashik, Maharashtra, India
2Pharmaceutics Department, Maratha Vidya Prasarak Samaj's College of Pharmacy, Nashik, Maharashtra, India
- *Corresponding Author:
- Poonam Mogal
Maratha Vidya Prasarak Samaj's College of
Pharmacy, Nashik, Maharashtra, India
Tel: 09590929155
E-mail: poonammogal@gmail.com
Received date: June 02, 2017; Accepted date: June 09, 2017; Published date: June 14, 2017
Citation: Mogal P, Derle D. Soluplus as a Potential Enhancer of Cefixime Biopharmaceutical Properties through Solid Dispersion Prepared by Different Pharmaceutical Interventions. Integr J Glob Health. 2017, 1:2.
Abstract
The present study was done for improvement of solubility and thereby bioavailability of Cefixime for its efficient antimicrobial activity by using amphiphilic Soluplus as a carrier. Different methods were used for enhancing the solubility of Cefixime like physical mix, kneading, microwave irradiation, solvent evaporation, lyophilization and spray drying. The plain drug and the prepared SD with various CFX to polymer ratios were characterized in terms of solubility, drug content, IR, DSC, SEM, XRD, dissolution, zone of inhibition and in vivo studies. A noticeable change in the dissolution rate was observed by in vitro dissolution studies in relation with pure CFX. The solid dispersion of CFX prepared showed following order in enhanced amorphous micellerization ratio like Spray drying>Lyophilization>Microwave irradiation>Solvent evaporation>Kneading method>Physical mixture>Pure drug. In addition, spray dried solid dispersion demonstrated remarkable highest inhibition of microbial zone than plain Cefixime (CFX) suggesting improvement in antibacterial activity of Cefixime by solid dispersion. Pharmacokinetic work by (n=6) rats demonstrate a significant difference (a=0.05) when the mean Cmax concentration from spray dried SD and Cefixime (CFX) were compared. The AUC was 1.9 times more in Cefixime (CFX) as SD administration, against the AUC obtained for the plain Cefixime (CFX) suspension. After SD dosing, Tmax (4 h) was different than that obtained in pure drug suspension (3 h). Thus, formulation of Cefixime (CFX) as SD, enhanced amorphous micellerization ratio of the drug and lead to significant (p=0.05) increase in absorption of Cefixime. Hence, Soluplus proved to be potential hydrophile for increasing oral bioavailability of Cefixime using spray drying technique and leading to improved health of patient with reduced dose of drug.
Keywords
Cefixime; Poor solubility; Soluplus; Solid dispersion; Micelles; Dissolutions
Introduction
Today, it is requisite to optimize the usage of older and novel in treating infections mainly because of the increase in drug resistance and scarcity of new for safeguarding global health. It is essential to increase the awareness to optimise a therapy for increasing the therapeutic outcome and to minimize the resistivity to the drug throughout the treatment. A clinical decision must be based on the exposure-response relationships [1-5].
The alteration in prescribed course of treatments constitute a major difficulty due to the older patent expired are more and more given to subjects now since emergent resistance posing a growing health challenge particularly for treating Gramnegative bacteria. So, it is important to encourage plans for beforehand management to lessen the increase of the infective microbes and maximizing its use for the safety of public health. It also helps in preventing lesser dosage to repress or reduce the possible increase of resistive mutants of microbes [6].
The frequent infective microbial agents are extracellular causing infection in tissues. Hence, interstitial fluid’s concentrations at the infection must be the main deciding factor for its effectiveness in body of patient. Concentrations of drug in serum/plasma of patient are better prognosticators of interstitial fluid levels compared to tissue homogenate. Tissue homogenates has mixture of intracellular, interstitial and vascular compartments with each other which leading to false approximation of the interstitial fluid concentration [7].
Globally, are highly prescribed medication in healthcare industry. They are completely unlike of other drug because the ultimate cell wall is located in the microbe and the result of the drug administration depends on the exposing of it to microbe in subject and does not depend on patient biology. So, it allows the study of exposing drug with its effect to get the optimum dose for drugs in scientific research.
For many categories of drugs the area under curve is related to effect, while effectiveness of beta-lactam rather depends a lot on the time the concentration of the stays above the minimal inhibitory concentration (T>MIC) of the microbe than its area under curve. It is reported that Cephalosporin’s concentrations must surpass the MIC for ≥ 50% of the dose ensuring the bacterial destruction with healing in respiratory tract infections [8].
It is important “to raise global AMR awareness, improve correct antimicrobial use, and develop the monitoring systems in the community for the sake of public health” and for safeguarding antimicrobials and in effect our society. The intestines have various commensal bacterias, boosting immune system and are essential for good health of the body. means those that acting against intestinal bacterias both essential and harmful one [9,10].
Using antibiotic may trouble the resistance of gastrointestinal flora (beneficial), giving symptoms like diarrhoea, abdominal pain, vomiting etc in the treated patient. Clostridium difficile, the pathogen mostly related with opportunistic growth, right after disruption of colonisation resistivity because of antibiotic treatment in the patient. The consequences of this are asymptomatic intestinal colonization, diarrhoea, colitis, and pseudo-membranous colitis or death which are causing serious concerns for public health in various parts of the world [11]. are often given to children in whom it alters the commensal proportion in the GIT, in effect showing antibioticassociated diarrhoea (AAD) [12].
The antibiotic treatment allows C. difficile cells to proliferate and producing the toxins leading to cause disease [13]. This infection spreading from person to person through the faeces or orally by the transmitting spores that prevail in atmosphere also spreading from patient to patient via physical contact of healthcare workers with infected patients [14].
These Gi infections are usually encountering infections in hospitalized patients with high antibiotic usage. Cefixime treatment is followed by side effects like severe diarrhoea and/ or Peudo membranous colitis. The dose employed in most of the cases is 400 mg which is higher and often prone to increase chances of opportunistic GI bacterial infections such as those caused by Clostridium difficile. Hence, it is necessary to decrease the dose of Cefixime used for the therapeutic purpose that may achieved by using different pharmaceutical interventions as used in the present work.
The dose reduction due to increased bioavailability can decrease the incidences of opportunistic GI bacterial infections such as those caused by Clostridium difficile. Another advantage is that the increased solubility may equate tablets to oral suspensions in terms of bioavailability thus with improved biopharmaceutical properties of Cefixime Where tablets are more convenient to supply-chain and dispense than suspensions.
Cefixime is a weak acid having pKa value 3.73 hence, lead to unionisation at acidulous pH and expands its assimilation in the stomach area. When given orally, it is gradually and deficiently absorbing from the GIT and thus, giving poor bioavailability around 40-50% because of inadequate aqueous solubility [15-19].
Cefixime, poorly bioavailable and extended spectrum cephalosporin antibiotic, was utilized in the present study which is commonly prescribed for treating respiratory and some urinary tract infection. Chemically, Cefixime is (6R, 7R)-7-[[(2Z) -(2-Amino- 4-thiazolyl) [(carboxymethoxy)imino] acetyl] amino]-3ethenyl- 8-oxo-5-thia-1-azabicyclo [4.2.0] oct-2-ene-2-carboxylicacid. Cefixime is observed to be very effective verses a wide scope of Gram-negative and some Gram-positive oxidative microscopic organisms. It has noteworthy P-lactamase stability, which is equivalent to parenteral cephalosporin, moderately prolonged half-life.
For the eventual transport of drug to the receptor site, two factors are mostly responsible i.e., solubility and permeableness of a drug. So, the solubility’s effect on a badly soluble drug’s bioavailability may present serious limitations in evaluating its true clinical efficacy [20,21].
Cefixime belongs to BCS class 2/4 the reason is unknown. Hence, in the present study, solubility enhancement methods are employed for increasing the poor solubility and in effect to check if its antibacterial activity is also increased or not [22-25]. Thus, the reason is unknown whether the poor oral bioavailability is solubility limited or permeability limited. Therefore, efforts were made for preserving this antibiotic by enhancing its poor water solubility with solubilizer.
Acceptable and appropriate formulation approaches are necessary in enhancing solubility and thereby bioavailability of poor aqueous soluble API. It has been established that the therapeutical efficaciousness of the potent API suffered by its poor biopharmaceutical properties [22-25]. Different solubility enhancement systems are employed to make up for their poor solubility and resulting moderate dissolution rate [26,27].
Different methods were used for enhancing the solubility of Cefixime like physical mix, kneading, solvent evaporation, microwave irradiation, lyophilization and spray drying. Soluplus is a graft copolymer having a PEG 6000 with 1 or 2 lateral chains made up of vinyl acetate at random co-polymerized with vinyl caprolactam. It is advisable to utilize Soluplus for solubility enhancement of poorly soluble drugs due to its amphiphilicity property [28,29].
This work was aimed to prepare and characterise solid dispersion of Cefixime with Soluplus. Dispersion of poorly soluble drug into hydrophilic carrier leads to increase in apparent solubility of drug which consequently increases its oral bioavailability in the patient’s body and consequently improving health of patient with less dose. This study here portrays the utilization of Soluplus as a solubilizer for poorly soluble Cefixime. This study likewise explores the impact of various techniques for dispersion of hydrophile on dissolution and solubility of Cefixime. The prepared solid dispersions were characterised for DSC, XRD, ATR/ IR and SEM for finding compatibility of drug and polymer in solid dispersion along with in vivo studies.
Materials
Cefixime IP (analytical grade) was kindly gifted by Glenmark pharmaceuticals, Sinnar, Nashik. Soluplus was got as gift sample from BASF, Turbhe, India. All other materials utilized were of analytical grade.
Methods
Preparation of Solid Dispersions (SDs)
Physical Mixtures (PM): Physical mixtures, in different ratios (1:1, 3, 5) were prepared by mixing Cefixime with Soluplus for four-five min in a mortar till a uniform mixture was obtained. The mixtures obtained were passed through sieve (# 80 mesh US standard) after which they were stored in a desiccator at normal temperature for further usage [30-32].
Kneading method (KD): Solid dispersions with various ratios like (1:1, 3, and 5) were prepared. Firstly, Soluplus was added up in the mortar, then little quantity of methanol was added together during trituration for acquiring similar uniformity like slurry. And then gradually drug was added in the slurry with triturating it for one hour. Air drying was carried out for it at 25°C for 24 h, powdered by sieving through sieve (# 80 mesh US standard) and after that it was kept in desiccator all over fused calcium chloride.
Solvent Evaporation method (SE): Solid dispersions in various ratios like (1:1, 3 and 5) were made by dissolving Cefixime (CFX) in Non-aqueous solvent whereas, Soluplus was made soluble in aqueous solvent. Dissolved drug solution was adding together to hydrophilic water solution by constant stirring and add-on rate of 7-9 ml/min. Nonaqueous along with aqueous phase were evaporated in an oven at 45-50°C for 5-6 h. The product was sieved through sieve (# 80 mesh US standard) and further, it was kept in desiccator at room temperature till further analysis by UV.
Microwave irradiation method (MW): The Cefixime (CFX) and Soluplus (1:1, 1:2, and 1:3) were accurately weighed. A homogeneous paste was made by adding Cefixime (CFX) and Soluplus with minimal quantity of solvents (Ethyl alcohol: H2O, 1:1 v/v) inside vessel. The paste developed was subjected to microwave irradiation for 7-8 min at power of 510 watt in a microwave (oven) synthesizer (CATA-R, catalyst systems, Pune, India). Just a single beaker was set at once inside the microwave synthesizer. The specimens were presented to microwave radiation for 7-8 min.
Temperatures of the contents were determined immediately after the microwave treatment using a thermometer (immersion type) that showed temperature of the samples between 45-50°C. The treated sample does not lose all water instead paste like consistency was maintained.
At that point, the beakers containing tests were kept up at 27- 29°C temperatures to cool down. The SDs were gathered and put in desiccator for 24 hours and afterwards the product was powdered utilizing mortar and pestle. The powdered product was sieved through sifter (# 80 mesh US standard) and put away in desiccators over combined calcium chloride. Microwave irradiation is often considered as hot melt treatment because it looked like melted system that requires cooling. As reported the glass transition temperature of Soluplus is 70°C. Hence in the reaction temperature (45-50°C which is less than polymer Tg) used for experiments, the system may have formed glassy state [33].
Lyophilization (LY): The Cefixime (CFX) and Soluplus (1:1, 1:2, and 1:3) were accurately weighed when Cefixime (CFX) was made soluble in methanol and polymer in aqueous solution. Methanoic solution was add up to water solution by uninterrupted stirring at the rate of 5-6 ml/min. The Nonaqueous solution (methanol) was removed by evaporation at room temperature. The sample were transferred into Ependorf tubes and frozen at -70°C for 3 h. The samples were then freeze dried (0.04 mbar vacuum for 24 h with increasing shelf temperature -30 to 0°C and a final drying for 2 h at +15°C) using lyophilizer (FreeZone Triad, Labconco, USA). The lyophilized product thus obtained was stored in desiccator at room temperature [34].
Spray drying (SY): Each solution for spray drying was prepared by adding different ratio of Cefixime and polymer (1:1, 1:2, 1:3) to 200 ml of methanol. It was exposed to ultrasonication by a bath sonicator for about 10 min [35-37]. After that spray drying was carried out in a lab spray dryer model LU-222 Advanced (Labultima, Mumbai, India) with the drying capacity of 1 L/h. Parameters set for spray drying were like using 4 ml/min flow rate, 80°C of inlet temperature, with 60-70°C of outlet temperature, and aspirator value of about 40 m3/h.
Drug content: Solid dispersions (equivalent to 10 mg of Cefixime) were made soluble in freshly prepared (pH 7.2 of 0.2M KH2PO4) in a 100-ml volumetric for determining drug content. Solution was fittingly diluted for taking UV-absorbance of the resulting solution and measuring it at wavelength of 288 nm against blank (phosphate buffer, pH 7.2) through UV-Visible Spectrophotometer. By plotting a graph of UV-absorbance versus concentration, the drug content was determined [30,31].
Solubility measurements of Cefixime (CFX): Explanation given by Higuchi and J Connors were used for solubility estimations of the samples. An excess quantity of solid dispersion was included 10 ml distilled water, which was taken in test tubes. The tests were sonicated for 1 h at room temperature. From that point, the topped test tubes were shaken at 25 or 45 ± 0.1°C for 24 h. In this manner, the suspensions were filtered through membrane filters and the separated solutions were collected in dry containers. The obtained solutions were suitably dried and analysed by Shimadzu UV 2501 PC, double beam spectrophotometer at 288 nm [30].
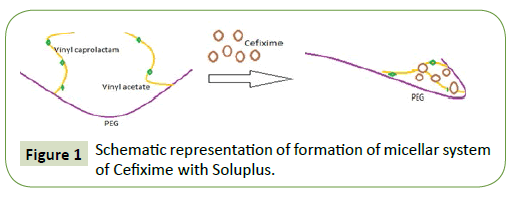
Turbidity: For determining critical micellar concentration of Soluplus in water, turbidity formation was used (Figure 1). For this, 5-100 mg/ml the Soluplus solution in water were prepared and its turbidity formation was assessed by UV-spectrophotometer at 500 nm [38].
Differential Scanning Calorimetry (DSC)
The possibility of any interaction between Cefixime and Soluplus amid arrangement of SD was evaluated via DSC examination and comparing it with that of pure Cefixime using DSC. DSC assessment was carried by differential scanning calorimeter (Lab Mettler Star SW 10). Heating of tests was done in sealed aluminium pan with heating a rate of 10°C/min over a temperature from 25 to 300°C using a nitrogen rate of flow at 50 ml/min.
X-ray Diffractometry (X-RD)
X-RD spectrum of pure Cefixime and binary systems were taken at normal temperature with the help of Bruker’s AXS D8-advance X-ray diffractometer. Irradiation of tests was carried out with mono-chromatized Cu KA2 emission at wavelength 1.5406 A0 and tests were mounted on zero-foundation test holder and subjected to a ceaseless scanning over angles of 3° to 80° 2θ at a stage size of 0.02°. The diffraction spectra were gathered with voltage of 40 kV and current of 35 mA individually. The examining rate was 20 min-1.
Scanning Electron Microscopy (SEM)
The topography of pure Cefixime and spray dried solid dispersion was looked under a scanning electron microscope (SEM; JEOL model JSM -6390LV) operating at an excitation voltage of 15 kV. The samples were mounted on a glass stub with doublesided adhesive tape and coated under vacuum with gold by ion sputtering using auto fine coater JSC 1600 (JEOL, Japan) prior to observation.
Attenuated Total Reflectance Spectroscopy (ATR) analysis
Supporting evidence for formation of solid dispersion can be acquired by IR spectroscopy. Attenuated total reflectance spectra of the drug samples were obtained on a Bruker Eco-ATR machine i.e., Attenuated total reflectance spectroscopy. For getting IR spectra, scanning was done over the wave number from 3600 to 400 cm-1.
Dissolution studies
These studies were carried out (100 mg drug equivalent) by utilizing USP 8-station dissolution test assembly (Lab India) utilizing USP type II device. Dissolution study was done in a 900 ml of pH 7.2 buffer at 37 ± 0.5°C at 100 rpm. 5 ml tests were withdrawn at time interims of 5, 10, 15, 20, 30, 45 min. The volume of dissolution medium was conformed to 900 ml to keep up sink conditions by supplanting every 5 ml aliquot pulled back with 5 ml of new pH 7.2 phosphate buffer. The drug’s concentration in tests was dictated by measuring absorbance at 288 nm. Aggregate percent drug release was ascertained at each time interim. Pure Cefixime was utilized as control for examination.
Antimicrobial studies
In-vitro antibacterial investigations of SDs were done by disk diffusion technique verses Gram-positive species S. aureus and Gram-negative species E. coli and P. aerugenosa. The activities were compared against pure Cefixime (CFX). For this studies Muller Hilton (MH) write out in full first time used agar medium was used as nutrient medium. The petri-plates were inoculated. Incubation of plates was done at 37°C for 24 h and the zone of inhibition was measured in mm by Zone-sizer scale [39].
In-vivo absorption study in wistar rats
Wistar-strain rats of either sex (180-220 g) were made available by the Animal House, college of Pharmacy, Nashik, India. They were housed under normal laboratory conditions. Rats were maintained on standard rodent chow and tap water. The Institutional Animal Ethical Committee (IAEC/Feb.2016/08) gave approval for the experimental methods.
The rats were kept with empty food however, but they were given clear reach to water 24 h earlier the day of the experiment. 3 rats group were utilized in the study. Random assignment of 6 rats to each treatment group was done. To individual group oral administration of Cefixime aqueous suspension, Cefixime- SDs and third group was control group, was done. Sample of Cefixime powder (8 mg), Cefixime-SDs (equivalent to Cefixime 8 mg), were mixed uniformly by accurately weighing and dispersing it into water (5 ml) for 30 s before to dosing. To rats, individual preparation was given utilizing oral gavage (an animal feeding needle). Blood samples (0.5 ml) were collected via tail vein at 30, 60, 120, 240, 360 and 720 min after oral administration into EDTA micro-centrifuge tubes. Centrifugation of samples was done at 10,000 rpm (15000 g) 10°C for 20 min where samples of plasma separated, and 0.5 ml of cold acetonitrile add up to the plasma sample for precipitating the protein. Centrifugation of the processed samples was done at 10,000 rpm 10°C for 20 min, and supernatant liquid was stored in refrigerator at -4°C until analyzed by HPLC [40].
HPLC analysis of plasma samples
Standard stock solution (100 μl) was diluted appropriately to get concentration of 10,20,30,40,50 μg/ml which were prepared and calibration curve was plotted by HPLC. 20 μl of plasma sample was put in into HPLC with splitless injection. Concentration of Cefixime in plasma was then further determined by calculating area under curve of chromatogram. The tailing factor was 1.21 with the retention time was 2.256 (% RSD of method precision is 0.1016% for 6 determinations).
HPLC (Binary HPLC pump provided with a UV visible 2000 detector, Waters 1525, Singapore) was utilized for the assessment of plasma samples. The separation was performed using a Cosmosil C18 packed column (150 mm × 4.6 mm, 5 μm) at 276 nm. The mobile phase consisted of a mixing of phosphate buffer having pH 4 ± 0.05 with methyl alcohol (50:50) operating at a flow rate of 1 ml/min with isocratic elution [41].
Data analysis
The average plasma Cefixime concentration profiles were produced. Statistical comparisons were performed using Graph pad prism for Windows Version 7. For assessing two mean values for statistical significance (at α=0.05 level of significance) was done by utilizing 2-tailed unpaired t test for samples with unequal variance where SOL-SD was analyzed with the Cefixime (CFX).
Results and Discussion
Drug content and solubility studies
The drug content was found to be consistent ranging from 98.86 ± 1.12% for triplicates. The critical miceller concentration of Soluplus in water was found to be 0.68 mg/ml while solution becomes gradually cloudy with bluish tinge when concentration of Soluplus was increased above CMC indicating formation of micelles structure with slightly evolution of heat at start of addition of Soluplus solution as shown by warming of test tubes [38].
The ability of micelles formation by Soluplus was bestowed on it due to presence of both hydrophilic (PEG-polyethylene glycol) and lipophilic (vinyl caprolactam/vinyl acetate side chains) part in its structure which give it amphiphilicity required for micelles formation in water [41].
As studied by Tanida et al., a distinct lessening in the endothermic reaction was because of decrement in the micelles’s dissociation as well as forming micelles of unimers when more micellar solution was added to the test.
Moreover, a diminutive thermal reaction was caused by the dilution of the micellar solution when more micellar solution was injected above the CMC. Thus, it can be concluded that solubilizer exists as micelles at the concentration employed for solid dispersion preparation. The system after keeping for 24 hours showed no phase separation indicating that micelles have formed stable saturated system of Cefixime.
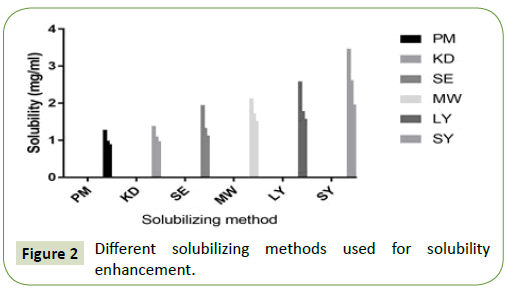
The solubility profile of physical mixture and solid dispersion in different proportions of Soluplus are depicted in Figure 2. Cefixime was slightly soluble in water. The amorphous micellar enhancement of pure Cefixime and its physical mixing with Soluplus in distilled water was less when compared to SDs prepared by other technique.
The amorphous micellar enhancement of solid dispersion made by spray drying method showed 7-fold rise in water than pure drug in contrast to this enhancement was 2.7-fold in physical mixing. It was clear that Soluplus had an enhancing outcome in the solubility of Cefixime. This may be due to micellar solubilization as reported by other similar studies using Soluplus [42].
In addition to this, the increase in free energy of dissolution due to amorphization as indicated by XRD study could be the reason for this enhancement. Hence, the combined effect might be responsible for the amorphous micellar enhancement of solid dispersion prepared by Soluplus. Soluplus is also known to form solid solution by providing matrix for the drug entrapment which along with micellar solubilization may be responsible for the amorphous micellar enhancement of solid dispersion.
For improving solubility or achieving supersaturated state depends on the fundamental interaction between drug and polymer are decisive which rely upon complex formation quality of Soluplus with Cefixime. Soluplus has lipophilic region of copolymers i.e., vinyl acetate and vinyl caprolactam having various binding sites for trapping hydrophobic Cefixime within them [38]. Surface active properties of Soluplus along with micelles formation as well as amorphization attainment by dispersions of solid using spray drying may be the reason for amorphization micellar enhancement of Cefixime.
SEM
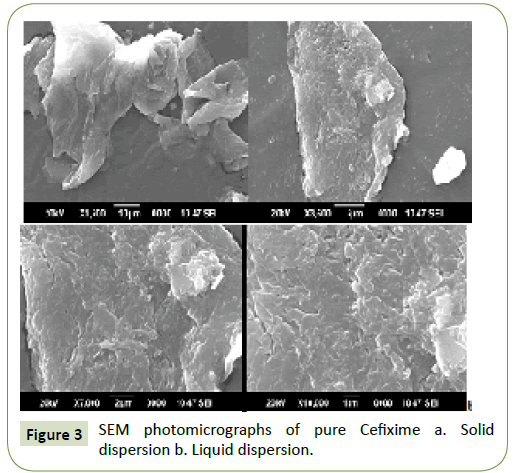
The surface structure of the Cefixime (CFX) and SOL-SD was evaluated using SEM and the photomicrographs are depicted in Figure 3. SEM photomicrograph of pure Cefixime (CFX) seems to be as planar-rough pointer-formed crystals of varied sizes with good-formed border. However, Cefixime (CFX)-SOL SD was appeared as irregular shaped particles of varied sizes with even surface. Interestingly, there was no crystalline structure of Cefixime (CFX) in SOL-SDs, showing the change of Cefixime (CFX) into a shapeless frame i.e., amorphous, which compare to the consequences of the DSC test.
Solid-State Characterization
DSC
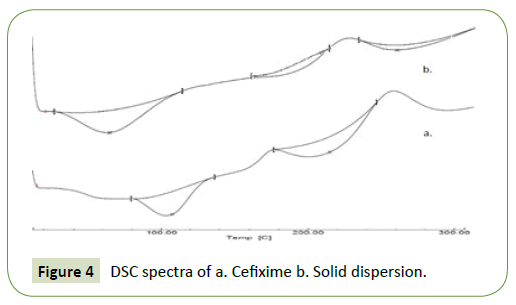
The thermic action of pure Cefixime (CFX) and solid dispersion with Soluplus are depicted in Figure 4. For pure Cefixime (CFX), DSC- thermogram demonstrated a phase transition endotherm at 215.64°C (with Hf;-138.41 Jg^o1); however, this endotherm was shifted to 235°C in the thermogram of spray dried SD with broadening illustrating the formation of solid dispersion of Cefixime and Soluplus where the enthalpy of fusion of CFX in the SD (-19.10 Jg^o1) is significantly lesser than its enthalpy of fusion when in pure state and is evident from the thermograms and thus Cefixime (CFX) and Soluplus were found to be compatible (Figure 3).
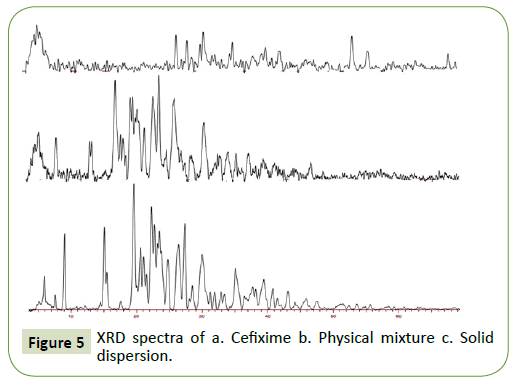
X-Ray Diffraction (XRD)
The X-ray powder diffraction images of Cefixime (CFX) and solid dispersion are illustrated in Figure 5. Pure Cefixime (CFX) indicates distinctive diffraction Brag peaks at 2θ positions of 5.89, 8.97, 19.55, 24.745, 26.36, 27.34, 31.88 and 35.112. Pure Cefixime (CFX) demonstrate pointed and acute peaks from 5°- 60° at 2θ positions indicating crystal form of Cefixime (CFX). X-RD performed on the physical blend dose not display Cefixime’s amorphous form instead it is addition of individual components.
However, for the SOL-SD, the discriminatory Cefixime’s Brag peaks were distinctly absent where halo pattern was seen for spray dried formulation showing the absence of crystallinity. Moreover, spray drying complex’s X-RD spectrum was seen distributed that was in opposite manner from that of pure Cefixime supporting the formation of new solid-state. The X-RD spectrum of spray drying SD is altogether diffuse, suggesting amorphous state of prepared SD.
ATR
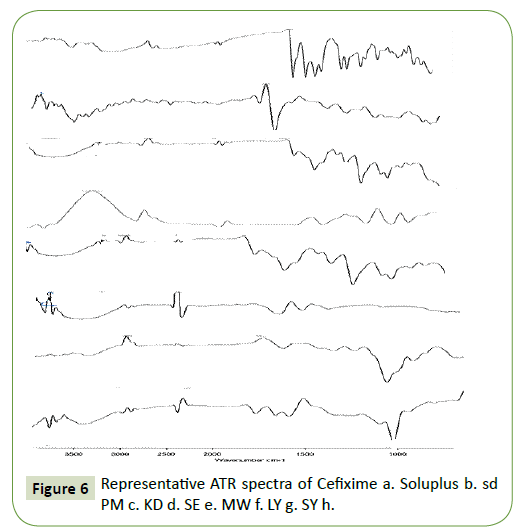
ATR spectroscopy was also carried out to assess interaction between Cefixime and Soluplus in solid phase. Hydrogen bond interaction may be anticipated in the hydroxyl function of Soluplus and the carbonyl group of Cefixime as per chemical point of view [43].
To assess any conceivable strong collaboration between the drug compound and hydrophile, ATR spectra of Cefixime, physical blends, and SDs were recorded and the outcomes are appeared in Figure 6. The ATR of SOL-SDs showed that a weakly OH extending vibration peak was seen at 3447 cm−1 and the peak of the Cefixime at 1761 cm−1 was no-show with just the C=O peak at 1728 cm−1 and 1646 cm−1 of the polymer was available. This finding proposed that Cefixime may have connected with Soluplus, for the most part by hydrogen holding. This sort of collaboration amongst API and hydrophilic carrier is an extra advantage for the SDs, since they can expand the strong dissolvability of the API into the hydrophilic polymer and additionally taking an attempt at restraining the crystallization of drug [44].
The ATR spectra discovered no interaction in drug with carrier. The functional groups are also not altered during mixing with carriers. The ATR spectra of Cefixime, Physical blend and solid dispersions were illustrated in Figure 5. Plain Cefixime displayed vibrational peaks of -NH2 primary amine at wave number 3287.89 cm−1, N-H stretch at 1661.43 cm−1, C-H at 1545.69 cm−1, C-N stretch at 1591.41 cm−1, N-O stretch at 1382 cm−1, Carbonyl stretch at 1761.16 cm−1.
Polymer Soluplus showed vibrational peaks of O-H at 3485.70 cm−1, C-H stretch at wave number 2993.54 cm−1, C=O (amide) stretch at 1646.89 cm−1, C=O (ester) stretch at 1728.97 cm−1, C-O at 1251.91 cm−1, 1095.85 cm−1, 1008.47 cm−1, C-N at 1357.07 cm−1. The ATR vibration of most importantly specified SDs were observed to be inside range with diminished intensity and also in physical blend showing nonappearance of any critical interaction in solid phase.
In vitro drug release
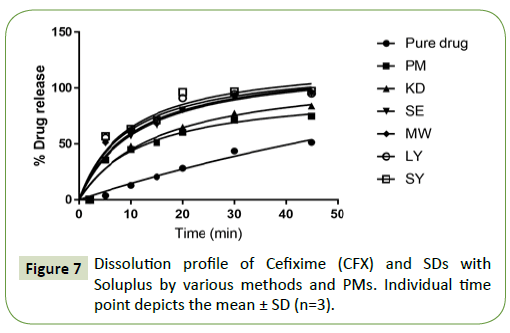
The percentage cumulative drug release profile of the different SDs of Cefixime (CFX) in PBS pH 7.2 and plain drug are analyzed in Figure 7. Overall, all the SDs were capable of enhancing the dissolution rate of Cefixime (CFX). This information is in correspondence with the rise in Cefixime amorphization micellar enhancement ratio in these formulations. Neat Cefixime (CFX) displayed a lowest dissolution rate and 43.52% drug release in 30 min and just achieved 51.37% after 45 min.
Physical mixtures released 74.75% of drug at 45 min. The formulation KD showed % cumulative release of 77.44% in 30 min pointing a 2-fold rise in drug dissolution in this period. The Sds prepared by methods like SE, LY exhibited a release of 94.90% and 95.02% respectively. The formulations with MW (96.45%) and SY showed like release curves with high dissolution rates as compared to the pure Cefixime (CFX). Therefore, the formulation SY with high % drug release of 97.36% was selected for advance work. Faster dissolution rate of Cefixime (CFX) in SDs might be a result of API solubilization by Soluplus in the dissolution medium due to different mechanisms.
The Addition of Soluplus to the medium increased the solubility of pure CFX in the dissolution medium as it was observed that the dissolution is significantly increased for the physical mixture as compared to pure Cefixime. Hence, micellar solubilization may be responsible for solubility enhancement of Cefixime andconsequently its release to dissolution medium.
This data is consistent with that reported by other Soluplus study on another drug i.e., ipriflavanone where physical mixing of drug and Soluplus significantly increased the solubility of drug by decreasing surface tension and increasing turbidity (micellar formation above CMC of Soluplus) [38].
Usually, the thermodynamic state of the API had more impact on the dissolution rate of the resulting SD. But this does not seem to be the case as the dissolution rate of PM is significantly greater than API alone.
Thus, amorphization may be contributing little for dissolution enhancement of Cefixime as can be seen by the dissolution profile for physical mixing and SD prepared by other methods. But in case of solvent evaporated binary system utilizing spray drying, by dissolving drug and polymer in solvent itself is decreasing its particle size where after spray drying giving amorphous particles as indicated by XRD study [45].
The slight upgrade of API dissolution when physically blended with the hydrophilic Soluplus could be ascribed to the neighborhood solubilization activity of the hydrophile functional inside the microenvironment or the hydrodynamic layer encompassing medication particles inside the early phases of the dissolution procedure or subsequently of the surface active alike properties of Soluplus that enhance the API wettability by lessening the interfacial surface strain between the water insoluble API particles and furthermore the dissolution medium, leading to improvement of the wettability and disintegration, ultimately dissolution of the API.
The slight upgrade of API dissolution when physically blended with the hydrophilic Soluplus could be ascribed to the vicinity of solubilization activity of the Soluplus functional inside the microenvironment or the hydrodynamic layer forming IM bonds between solute and solvent (hydration energy).
As indicated by XRD study, the sd is exhibiting amorphous form hence out of the two basic forces for dissolution (total energy is the enthalpy of solution): energy absorbed to overcome the lattice energy of a crystalline substance, and energy released when forming IM bonds between solute and solvent (hydration energy); the contribution of this term is zero and the enthalpy of solution is only dependent on the hydration energy.
The SDs prepared by kneading, illustrated marginally greater increase in Cefixime dissolution contrasted with the physical blends. The observed augmentation in dissolution is inferable from the increase in inside the API- hydrophile contact surface therefore of improved mechanical treatment [46].
The SDs arranged by microwave illumination represented more improvements in disintegration and dissolution of Cefixime contrasted with the physical blends and SE, KD which could be credited to the enhanced interaction between Cefixime and Soluplus using these methods. The microwave irradiation duration was chosen on the premise of preparatory work where distinctive circumstances running from 4 to 8 min were tried for the SDs. The ideal illumination planning was 7-8 min. The more irradiating time prompts lessening in dissolution times that may be because of increased interactions due to microwave radiation (electromagnetic radiation).
The dispersion of solid made by spray drying demonstrated noticeable increment in Cefixime (CFX) dissolution than other techniques. This improvement may be due to amorphization conferred on it by spray drying techniques as evident from XRD data where it showed decrease of the crystallinity taking after dispersion in carrier [47]. The checked increment inside the dissolution can be referable to the creation of solubilized Cefixime inside the lyophilized systems with decreased particle size when the Soluplus took the Cefixime into the dissolution medium, helping in quick dissolving action [48].
The Cefixime’s dissolution enhanced significantly (t-test; P<0.05) in the spray drying-solid dispersion. It is well established in literature that amorphous state of drug exhibit faster dissolution and a supersaturation regime as compared to crystal forms and spray drying method lead to formation of amorphous state as noticeable from XRD, DSC, SEM results which could be accountable in improving the dissolution rate of Cefixime (CFX) [49,50].
This enhanced cumulative outcome could be because of presence of Soluplus, a hydrophilic-amphiphilic carrier causing wetting of drug particles in both methods and also formation of smaller particles giving enlarged surface area within SDs formed by spray drying method were accountable for increased solubility and dissolution. SDs formed using spray drying method demonstrated all API release with highest solubility than other techniques [51,52].
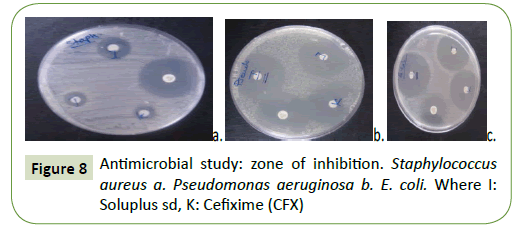
Antimicrobial studies
The antibacterial activity of SDs of Cefixime (CFX) with Soluplus verses Gram-positive (S. aureus) and Gram-negative (E. coli) species was tested using disk diffusion method and with the plain Cefixime (CFX). The zone of inhibition as it can be seen in Figure 8. These activities disclosed that SDs of Cefixime (CFX) have illustrated greater antibacterial activity (Table 1) than plain Cefixime (CFX). Nonetheless, Cefixime (CFX)-SOL spray dried SD has demonstrated remarkable highest inhibition of microbial zone than plain Cefixime (CFX) as well as other SDs. The higher antimicrobial activity of Cefixime (CFX)-SOL spray dried may be because of the quality of Soluplus to freeing the drug promptly from the solid dispersion [53].
| Sample name | E. coliATCC 25922 | Pseudomonas aeruginosaATCC 27853 | Staphylococcus aureusATCC 25923 |
|---|---|---|---|
| Cefixime | 33mm | 30mm | 12mm |
| Gentamycin (Control) | 20mm | 27mm | 27mm |
| Solid dispersion | 35mm | 31mm | 16mm |
Table 1: Antimicrobial study (Zone of inhibition).
| Parameter | Cefixime (CFX) | SD |
|---|---|---|
| AUC0-12 hμg·h/mL | 11.28 | 21.77 |
| Cmaxμg/mL | 1.365±0.01607(limit of detection (LOD:0.225µ/ml) | 2.988±0.04512 |
| Tmaxh | 3 | 4 |
Table 2: Pharmacokinetic parameters following oral administration of solid dispersion and Cefixime (CFX) (n=6).
In vivo absorption study in wistar rats
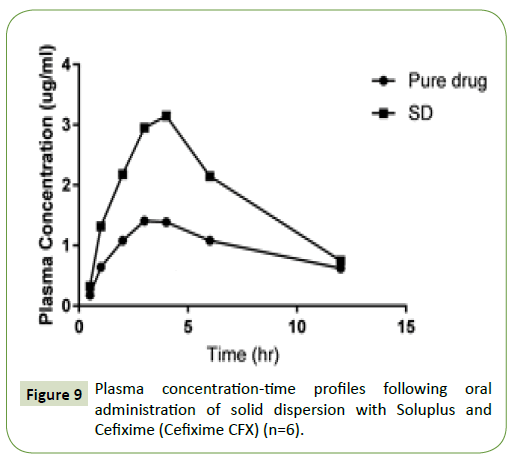
The plasma concentrations vs. time profiles are depicted in Figure 9, with the in vivo absorption parameters are summed up in Table 2. The administration of drug orally of the aqueous suspensions of Cefixime gave the mean Cefixime plasma concentrations. The AUC was 1.9 times higher of Cefixime when administered as SD, than for the aqueous Cefixime suspension. The Tmax (4 h) after SDs dosing was distinct than the Tmax of plain drug suspension (3 h). The mean residence time (MRT) of Cefixime was also increased (3.8 ± 0.82 h) in solid dispersion than pure Cefixime (MRT 2.3 ± 0.64 h) which pointed the improvement in metabolic stability of Cefixime in SD in comparison to pure drug. These outcomes disclosed that preparation of Cefixime as SDs brought significant (p=0.05) increment in absorption of Cefixime, than that from the plain Cefixime suspensions. The improved oral bioavailability of SD has occurred might be through the smaller drug particle formation with enlarged surface area as well as with diminished dispersal layer thickness that leads to quicker absorption through the duct wall.
Figure 9:Plasma concentration-time profiles following oral administration of solid dispersion with Soluplus and Cefixime (Cefixime CFX) (n=6).
Conclusion
We investigated the consequence of graft copolymer, Soluplus on solubility and dissolution of Cefixime while work was focused on employing simpler and inexpensive methods viz.; kneading, microwave irradiation and freeze-drying method, spray drying method.
This work gave understanding in the capability of different pharmaceutical interventions in improving the solubility of Cefixime (Cefixime CFX). The spray drying improved amorphization micellar enhancement ratio greatly, whereas solvent evaporation, lyophilization and microwave irradiation also provided significant improvement in it of Cefixime (CFX). It can be concluded that spray drying method demonstrated better solubility and dissolution improvement for Cefixime than other methods. A first ruptured effect to a greater extent than 50% in the first 5 min as well as 80% dissolution within 30 min was seen in these conditions. In this way, the previously mentioned framework can be considered as productive for upgrading the dissolution of Cefixime with the likelihood of enhancing the bioavailability and hence lessening the dosage of the medication. This decreasing the dose would decrease the incidences of opportunistic GI bacterial infections such as those from Clostridium difficile.
Dispersal of Cefixime (CFX) in solid dispersion by spray drying with Soluplus gave significant enhancement in AUC (0−t), Cmax, and thus in effect oral bioavailability of poorly soluble drug.
Formulation approach involving incorporation of hydrophilic polymer, Soluplus, increases its antibacterial activity by enhancing its solubility, indicating improvement in its antibacterial use for treating infections. This work underlines the significance of handling different solubility enhancing techniques to various drug delivery obstacles like solvency, dissolution and its antibacterial activity, to accomplish noteworthy oral bioavailability improvement of Cefixime (CFX). From the work it was presumed that spray drying demonstrated better dissolvability and dissolution when contrasted with physical blend and unadulterated API. This showed dissolvable solvent treatment and incorporation of hydrophilic polymer adds to upgraded dissolution of inadequately water soluble drug which subsequently expands its oral bioavailability and therapeutic efficacy.
Acknowledgements
The authors would like to thank the Sophisticated Analytical Instrumentation Facility (SAIF) at Cochin University, Kochi for the PXRD, SEM studies. I am also thankful to the Glenmark pharmaceuticals, Sinnar, Nashik and BASF, India for providing gift samples needed for the research work.
References
- Oneill J (2016) Tackling drug-resistant infections globally: final report and recommendations. The review on antimicrobial resistance.
- Antimicrobial Resistance (2014) Global Report on surveillance. WHO Library.
- Oneill J (2015) Securing new drugs for future generations: the pipeline of , the review on antimicrobial resistance.
- Outterson K, Powers J, Seoane-Vazquez E, Rodriguez-Monguio R, Kesselheim A (2013) Approval and Withdrawal of New and Other Antiinfectives in the U.S., 1980-2009. J Law, Med Ethics, pp: 688-696.
- Theuretzbacher U, Van Bambeke F, Canton R, Giske C, Mouton J, et al. (2015)Reviving old . Journal of Antimicrobial Chemotherapy. J Antimicrob Chemother 70: 2177-2181.
- Moutona J, Ambrosed P, Cantone R, Drusanof G, Harbarthg S, et al. (2011) Conserving for the future: New ways to use old and new drugs from a pharmacokinetic and pharmacodynamic perspective.Drug Resistance Updates 14:107-117.
- Craig W (1998) Pharmacokinetic/Pharmacodynamic Parameters: Rationale for Antibacterial Dosing of Mice and Men.Clinical Infectious Diseases 26:1-12.
- Yu V, Chiou C, Feldman C, Ortqvist A, Rello J, et al. (2003) An International Prospective Study of Pneumococcal Bacteremia: Correlation with In Vitro Resistance, Administered, and Clinical Outcome.Clinical Infectious Diseases 37:230-237.
- Cash H,Hooper L (2005) Commensal Bacteria Shape Intestinal Immune System Development.71: 77-83.
- Tlaskalova-Hogenovaa H, Epankovaa R, Hudcovica T, Tu Ckovaa L, Cukrowskab B, et al. (2004) Review Commensal bacteria (normal microflora), mucosal immunity and chronic inflammatory and autoimmune disease.Immunology Letters 93: 97-108.
- Berrington A (2004) Impact of mandatory Clostridium difï¬ÂÂcile surveillance on diagnostic services. J Hosp Infec 58: 241-242.
- Hayes S, Vargas A (2016) Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Explore: The Journal of Science and Healing.
- Blondeau JM (2009) What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 63:238-234.
- Johnson A (2010) New for selective treatment of gastrointestinal infection caused by Clostridium difficile. Expert Opin Ther Patents,p: 20.
- Martindale (2002) Complete Drug Release. 33rd edn by Sean L Sweetman, Published by Pharmaceutical Press, UK, 2002: 166.
- Clark Analysis of Drugs and Poisons (2004) 3rd edn.In: Anthony CM, Osselton DM, Widdep B. Pharmaceutical Press of Great Britain, pp: 763-764.
- Stone J, Iinong G, Andrews J, Wise R (1989)Cefiximein-vitro activity,pharmaacokinetics and tissue penetration. Journal of Antimicrobial Chemotherapy 23:221-228.
- Mamzoridi K, Kasteridou N, Peonides A, Niopas I (1996) Pharmacokinetics of Cefixime in Children with Urinary Tract Infections after a Single Oral Dose. Pharmacology and Toxicology 78:417-420.
- Brittain D, Scully B, Hirose T, Neu H (1985) The pharmacokinetic and bactericidal characteristics of oral Cefixime. Clinical Pharmacology & Therapeutics 38:590-594.
- Thomas H, Bhattachar S, Hitchingham L, Zocharski P (2006) Review: The road map to oral bioavailability: an industrial perspective. Expert Opin Drug Metab Toxicol 2:591-608.
- Lipins C (2002) Poor aqueous solubility-an industry wide problem in drug discovery. Am pharm Rev 5:82-85.
- LoÈbenberga R, Amidon G (2000) Review article: Modern bioavailability, bioequivalence and biopharmaceutics classification system. New scientific approaches to international regulatory standards. European Journal of Pharmaceutics and Biopharmaceutics 50: 3-12.
- Bergstrom C, Andersson S, Fagerberg J, Ragnarsson G, Lindahl A (2013) Is the full potential of the biopharmaceutics classification system reached? Eur J Pharma Sci,pp: 1-8.
- Dahan A, Miller J, Amidon G (2009) Prediction of Solubility and Permeability Class Membership: Provisional BCS Classification of the World’s Top Oral Drug. The AAPS Journal 11:740-746.
- World Health Organization (WHO) (2006) Proposal to waive in vivo bioequivalence requirements for WHO Model List of Essential Medicines immediate-release, solid oral dosage forms. Technical Report Series.
- Pinnamanenei N, Das N (2002) Formulation approaches for orally administered poorly soluble drugs. Pharmazie 57:291-300.
- Loftsson T, Hreinsdottir D, Masson M (2010) Evaluation of cyclodextrin solubilization of drugs. Int J Pharm 302:18-28.
- BASF (2017) Pharma Ingredients and Services, pp: 1-8.
- Raymond C, Paul J, Sian C (2005) Handbook of pharmaceutical Excipients. 5th edn. PhP Pharmaceutical Press.
- Teresa M, Victoria M, Gloria E (2002) Characterization and solubility study of solid dispersions of flunarizine and polyvinyl pyrrolidon. IL Farmaco 57:723-727.
- Sav A, Desai H, Meer T, Purnima A (2012) Solubility and Dissolution Rate Enhancement of Curcumin Using Kollidon VA64 by Solid Dispersion Technique.Int J PharmTech Res 4:1055-1064.
- Mahaparle P, Gudsoorkuar V, Gajeli G, Kuchekar B (2006) Studies on Solid Dispersions of Meloxicam.Int JPharmEducRes40:241-245.
- Wen X, Tan F, Jing Z, Liu Z (2004) Preparation and study of the 1: 2 inclusion complexes of Carvedilol with β-cyclodextrin. J Pharm Biomed Anal 34: 517-5230.
- Nagarsenker M, Joshi M (2005) Celecoxib-cyclodextrin systems: characterization and evaluation of in vitro and in vivo advantage. Drug Dev Ind Pharm 31:169-178.
- https://www.allfordrugs.com/spray-drying/
- Aulton ME, DryingD (2002) In: Pharmaceutics-The Science of Dosage Form Design. 2nd edn. Churchill, Livingstone, pp: 389-390.
- Badr-Eldin M, Ahmed A, Ismail R (2013) Aripiprazole-cyclodextrin binary systems for dissolution enhancement: effect of preparation technique, cyclodextrin type and molar ratio. Iran J Basic Med Sci 16:1223-1231.
- Tanida S, Kurokawa T,Sato H,Kadota K, Tozuka Y (2016) Evaluation of the micellization mechanism of an amphipathic graft copolymer with enhanced solubility of Ipriflavone chem. Pharm Bull 64:68-72.
- Saindane DS (2010) Dissolution improvement of poorly water soluble drug by freeze drying with pvp k-30. 1:149-162.
- Setthacheewakul S, Mahattanadul S, Phadoongsombut N, Pichayakorn W, Wiwattanapatapee R (2010) Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin and absorption studies in rats. Eur J Pharm Biopharm76: 475-485.
- Jain N, Jain H, Jain P (2014)Validated Stability Indicating RP-HPLC Method for Simultaneous Estimation of Ofloxacin and Cefixime in their Combined Dosage Form. Sci Technol Arts Res J 3: 93-98.
- Dian L, Yu E, Chen X, Wen X, Zhang Z, et al. (2014) Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res Lett 9:684-686.
- Verheyen SB, KingetN, van den MooterR (2002) Mechanism of increased dissolution of diazepam and temazepam from polyethylene glycol 6000 solid dispersions. Int J Pharm 249:45-58.
- Riekes M, Kuminek G, Rauber G, Bortoluzzi A, Stulzer HK (2014) HPMC as a potential enhancer of nimodipine biopharmaceutical properties via ball-milled solid dispersions. Carbohydr Polym 99: 474-482.
- Dangprasirt P, Ritthidej G (1995) Development of diclofenac sodium controlled release solid dispersions by spray drying using optimization strategy I. Drug Dev Ind Pharm 21: 2323-2337.
- Fernandes C, Veiga F (2002) Effect of the hydrophobic nature of triacetyl-β-cyclodextrin on the complexation with nicardipine hydrochloride: physicochemical and dissolution properties of the kneaded and spray-dried complexes. Chem Pharm Bull 50:1597-1602.
- Zingone G, Rubessa F (2005) Preformulation study of the inclusion complex of warfarin-β-cyclodextrin. Int J Pharm 291:3-10.
- Betageri G, Makarla K (1995)Enhancement of dissolution of glyburide by solid dispersion and lyophilization techniques. Int J Pharm 126:155-160.
- Hancock B, Parks M (2000) What is truesolubility advantage for amorphouspharmaceuticals? Pharm Res17: 397-403.
- Friedrich H, Nada A, Bodmeier R (2005)Solid state and dissolution ratecharacterization of Co-groundmixtures of nifedipine andhydrophilic carriers. DrugDevInd Pharm 31: 719-728.
- Vasconcelos T, Sarmento B, Costa P (2007) Solid dispersions as strategy to improve oral bioavailability of poor water-soluble drugs. Drug Discov Today 12: 23-24.
- Duncan C (2002) Review the mechanisms of drug release from solid dispersions in water-soluble polymers. Int J Pharmac 231:131-144.
- Aleema O, Kuchekara B, Pore Y, Late S (2008) Effect of β-cyclodextrin and hydroxypropyl β-cyclodextrin complexation on physicochemical properties and antimicrobial activity of cefdinir. Journal of Pharmaceutical and Biomedical Analysis47: 535-540.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences