Proper Physical Exercise may be in Favour of Joint Health: Mechanical Stress in Combination with Verapamil can Raise the Production of Type II Collagen and Suppress the Production of MMP-1, MMP-13 in Human Osteoarthritic Chondrocyte/Agarose Constructs.
Dan L1, Yan S1, Jian-Qiang LV2, Yong-Qian F3, Dong-Hui H3, Wei-Long L3, Hai-Min S3, Hu-Ji X4 and Jian-Long G1*
1Department of Immunology and Rheumatology, Huadong Hospital, Fudan University, Shanghai 200040, China
2School of Kinesiology, Shanghai University of Sport, Shanghai 200433, China
3Department of Orthopedic Surgery, Huadong Hospital, Fudan University, Shanghai 200040, China
4Department of Rheumatology, Changzheng Hospital, Second Military Medical University, Shanghai 200003, China
- *Corresponding Author:
- Jian-Long G
Department of Immunology and Rheumatology Huadong Hospital, Fudan University, Shanghai 200040, China
Tel: +862162483180
E-mail: jianlong_guan@126.com
Received date: December 24, 2018; Accepted date: January 06, 2019; Published date: January 16, 2019
Citation: Dan L, Yan S, Jian-Qiang LV, Yong-Qian F, Dong-Hui H, et al. (2019) Proper Physical Exercise may be in Favour of Joint Health: Mechanical Stress in Combination with Verapamil can Raise the Production of Type Collagen and Suppress the Production of MMP-1, MMP-13 in Human Osteoarthritic Chondrocyte/Agarose Constructs. J Physiother Res. 2019, Vol.3 No.1:1.
Copyright: © 2019 Dan L, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To Investigate the effect of mechanical stress and verapamil on type collagen synthesis in human Osteoarthritic chondrocyte/agarose constructs, and whether these two factors interactively promote type collagen synthesis.
Design: Mechanical stress of 24 kPa was applied to osteoarthritic chondrocyte/agarose constructs for 0.5 h, 1 h and 2 h. Pre-stress treatment with 40 μmol/L verapamil was added and used to test whether it can interactively influence type collagen metabolism. RT-PCR, ELISA and Western Blot were performed to measure the expression of MMP-1, MMP-3, MMP-13 and type collagen. Statistical significance was assessed by ANOVA test.
Results: Mechanical stress of 24 kPa resulted in a time-dependent increasing of the production of type collagen while decreasing of MMP-1, MMP-13 from chondrocytes, but no significant trend was observed in the expression of MMP-3. Whether or not combined with mechanical stress, verapamil (40 μmol/L) can inhibit the expression of MMP-1, MMP-3 and MMP-13 (P<0.05), and promote the expression of type collagen (P<0.05). However, mechanical stress can enhance its anabolic effects on type collagen than verapamil alone.
Conclusions: Mechanical stress, either independently or in combination with verapamil treatment, could raise the production of type collagen in human osteoarthitic chondrocytes in this study predict that proper protective effect for Articular cartilage in healthy Population
Keywords
Osteoarthritis; Chondrocyte; Type collagen; Mechanical stress; Verapamil
Abbreviations
OA: Osteoarthritis; PG: Proteoglycan; MMPs: Matrix Metalloproteinases; EMC: Extracellular Matrix; FRZB: Firzzled-related protein; RT-PCR: Reverse Transcriptase-Ploymerase Chain Reaction; ELISA: Enzyme- Linked Immunosorbent Assay.
Introduction
Osteoarthritis (OA) is the most common bone and joint disease in the ageing population, and seriously affected the quality of their life [1,2]. Along with the destruction of cartilage and formation of osteophytes, joint cannot afford tolerable pressure as previously [3]. The pathogenesis of osteoarthritis is still controversial. Presently, it is widely accepted that under the action of mechanical stimulation, the chondrocytes secrete proteinase or inflammatory mediators, which break the balance of chondrocytes extracellular matrix synthesis and metabolic, leads to the occurrence of OA.
Cartilage extracellular matrix is mainly composed of type collagen and proteoglycan (PG). Type collagen forms fiber network, maintain the shape of the tissue and the stability of chondrocytes in the environment [4,6]. During the onset and progress of OA, chondrocytes produce excessive amounts of proteolytic enzymes, namely matrix metalloproteinases (MMPs), in response to a milieu of pro-inflammatory cytokines [4,5]. MMPs are also important in extracellular matrix (ECM) homeostasis [7]. The breakdown of type collagen is believed to be initiated by members of the MMP family, such as MMP-1, MMP-3 and MMP-13, which can destruct the integrity and function of articular cartilage [8-10].
Mechanical pressure is also a significant regulator in chondrocyte metabolism. High incidence of osteoarthritis was observed in obesity and athletes, which reveals a major role of mechanical pressure [11]. The mechanism of mechanical signal transmission is still unclear. Some studies suggested that mechanical stress cause the deformation of cartilage, which leads to the changes of extracellular ionic composition and the load potential [12].
Intracellular Ca2+ is a potent modulator involved in regulating numerous cellular activities. Considerable evidence indicates that the control of gene expression by Ca2+ occurs primarily via mechanisms that recognize and “decode” the amplitude, duration, frequency, and spatial properties of Ca2+ pulses typically initiated by extracellular stimuli [13]. Ping guan-Murphy et al. reported that Ca2+ signaling in chondrocytes is stimulated by compression via cyclic cell deformation [14]. Verapamil is used at low concentrations to selectively block L-type calcium channels, although it also blocks T-type channels at intermediate concentrations [15]. Takamatsu A and his colleagues found verapamil (up to 50 μmol/L) inhibited Wnt/beta-catenin signalling, and protected cartilage from degradation [16]. Under our experimental conditions, the effect of verapamil at the lower and intermediate doses should reflect a selective blocking of L-type calcium channels, so chondrocytes was treated with verapamil (40 μmol/L).
We hypothesized that verapamil might help mechanical stress stimulate chondrocytes biosynthesis of type collagen. Chondrocyte/agarose constructs were applied in this research. We firstly compared the effects of different duration of mechanical stress to the secretions of MMP-1, MMP-3, MMP-13 and type collagen. We also measured the effects of verapamil combined with IPH on biosynthesis of MMP-1, MMP-3, MMP-13 and type collagen in chondrocytes. To investigate the effect of mechanical stress and verapamil on type collagen metabolism of chondrocytes, and provide new ideas on osteoarthritis therapy. Currently, the treatments of osteoarthritis are still limited. Non-steroidal anti-inflammatory drugs are mainly used to early in the disease for pain control, and joint replacement is taken for severe patients, so more studies are required to find new ways to control osteoarthritis.
Subjects and Methods
Patients and cartilage source
Chondrocytes were obtained from 9 OA patients (aged 66-75 years) who had a total knee replacement operation at the Orthopedic Surgery, Huadong Hospital, Fudan University from September 1st, 2015 to October 10th, 2015. All patients were diagnosed with OA according to the American College of Rheumatology classification criteria [17]. All participants were informed consent; the experiment was approved by the ethics committee of Huadong Hospital, Fudan University.
Antibodys and reagents
Type collagen enzyme (GIBCO, USA), ELISA kits (Bio-Swamp, China), antibodies of MMP-1 (15000), MMP-3 (1500), MMP-13 (13000) and type II collagen (15000) (Abcam, England), β-actin (11000, CST, USA), HRP-labeled Goat Anti-Rabbit IgG, HRPlabeled Donkey Anti-Goat IgG, HRP-labeled Goat Anti-Mouse IgG (Beyotime, China), Trizol (Invitrogen, USA), SYBR Green (Thermo, USA), SDS (solarbio, China), NC membranes (Millipore, USA).
Isolation and culture of chondrocytes
Cartilage was obtained from operating room within 4 hours after the surgery. Chondrocytes were isolated from tibial plateau. After being cleaned of bone and fiber, cartilage was diced into approximately 1 mm3 pieces. Nine patients were divided into three groups to RT-PCR, ELISA and Western blot analysis. Chondrocytes from each patient were cultured respectively at a density of 2 × 106 cells/ml in feed medium which consists of DMEM, 10% FBS and 1% of mixed norvancomycin hydrochloride (10 mg/l, wt/vol in NS), amikacin sulfate injection (10 mg/l, wt/vol in NS), and fluconazole injection (0.1 mg/l, wt/vol in NS). Chondrocytes were cultured as a monolayer in humidified atmosphere at 37°C in 5% CO2. Medium was changed every two days.
Preparation of chondrocyte/agarose constructs
Agarose hydrogels were prepared by mixing four parts of 2.5% low melting point agarose (wt/vol in distilled water) with one part of 5 × DMEM/F12 containing 10% FBS, 50 mM Hepes, 500 μg/ml nor vancomycin hydrochloride, 500 μg/ml amikacin sulfate injection, and 5 μg/ml fluconazole injection [18]. The agarose hydrogels were liquefied by heating in boiling water until they were homogeneous at temperature below 40°C. Chondrocytes were then centrifuged and suspended in the agarose solution at a density of 2 × 106 cells/ml. The cells were counted by the Trypan blue exclusion method using a hemocytometer, and 8 ml of the mixture was placed into a culture dish with a diameter of 60 mm. The chondrocyte/ agarose constructs were then turned into gel in room temperature and punched to obtain cylindrical gels (5 mm in diameter and 3 mm in height) which were placed into the wells of a BiopressTM compression plate (Flexcell international). Subsequently, 1.5 ml DMEM/F12 culture medium containing 10% FBS, 100 μg/ml nor vancomycin hydrochloride, 100 μg/ml amikacin sulfate injection, and 1 μg/ml fluconazole injection was added to each well. The cell/ agarose constructs were maintained in culture for 24 h in 5% CO2 at 37°C.
Application of mechanical stress to chondrocyte/agarose constructs
All samples were subjected to load with a FX-5000 TM Flexcell® Compression Plus™ System (Flexcell International). Chondrocyte/agarose constructs were placed into BiopressTM compression plates. Each plate contains six constructs. A proper height was set up for the constructs. The strain regimen was programmed using the Flexcell software. Compression of 24 kPa was applied to the cell/agarose constructs undering a static waveform for 0.5 h, 1 h and 2 h. Four plates were pressed at the same time, if three were in each group, the fourth one need to be filled with empty plate to ensure that the pressure is balanced. After compression, the chondrocyte/ agarose constructs were further cultured for 24 h in 5% CO2 at 37°C. In the end, the chondrocyte/agarose constructs were snap frozen in liquid nitrogen and stored at -80°C fridge for further examination. Control group was also placed into the incubator, but no pressure was applied.
Verapamil treatment
The experimental group of primary OA chondrocytes was treated with verapamil (40 μmol/L). The verapamil solution (5 mg in 2 ml distilled water) was diluted with 10 ml saline and 120 μl of the diluted solution and added directly to the culture medium of the wells in the Bio press compression plates before compression.
Reverse transcriptase-polymerase chain reaction (RT-PCR) analysis
Total RNA was extracted from chondrocytes in agarose gels with Trizol in accordance with the manufacturer’s protocol. RTPCR was performed by SYBR Green Mix. Primers are shown below. The experimental data were analyzed by ABI prism 7300 SDS software. The signal levels for the MMP-1, MMP-3 AND MMP-13 and type collagen mRNA was expressed as ratios to the signal levels for Beta-ACTIN mRNA. ΔCt=Ct median mRNAs-Ct median β-actin. Expression fold changes were calculated using the 2-ΔCt method. Each sample was analyzed in triplicate to ensure accuracy.
ELISA (Enzyme-linked immunosorbent assay analysis)
The MMP-1, MMP-3, MMP-13 and type collagen standard were reconstituted and diluted as directed in the standard’s instruction. To initiate the assay, the assay diluent RD1-52 (100 μl/well) containing a buffered protein base was added to each well. The standard and the undiluted sample (100 μl/well) were then added, and the wells were incubated for 30 min at 37°C. After washing for five times with wash buffer by an auto washer, the plates were incubated with 200 μl/well of mouse monoclonal antibody against MMP-1, MMP-3, MMP-13 and type collagen conjugated to horseradish peroxidase for 2 h at room temperature on the shaker. After washing as described above, Chromogen Solution A 50 μl and Chromogen Solution B 50 μl were added, and the plates were incubated at 37°C for 15 min in the dark. The reactions were terminated by adding 50 μl/well of stop solution. Then, the absorbance at 450 nm was measured by a microplate reader (Lab systems Multiskan MS).
Western blot analysis
Chondrocyte/agarose constructs were washed in ice-cold phosphate-buffered saline, and pulverized in 5 × pressure buffer containing SDS. The chondrocyte/agarose lysis solution was heated at 95°C for 10 minutes and centrifuged at 12,000 rpm for 10 minutes. Supernatants were collected and protein concentration was determined. Equal amounts of protein (20 μg) were separated by 10% SDS-PAGE and transferred to NC membranes. Membranes were blocked in 5% nonfat milk for 1 h at room temperature, and then washed three times with TBS plus 0.1% Tween-20. The binding was detected by Western Blotting Chemiluminescence method, in accordance to the manufacturer's instructions.
Statistical analysis
The results were presented as the mean ± SEM (standard error of the mean). Statistical significance was assessed by ANOVA using SPSS 17.0 Version for Windows, two-way ANOVA with Bonferroni post-test (SPSS Inc., Chicago, IL, United States). P<0.05 was considered as statistically significance for all tests.
Results
Mechanical stress on type collagen, MMP-1, MMP-3 and MMP-13 mRNA expression
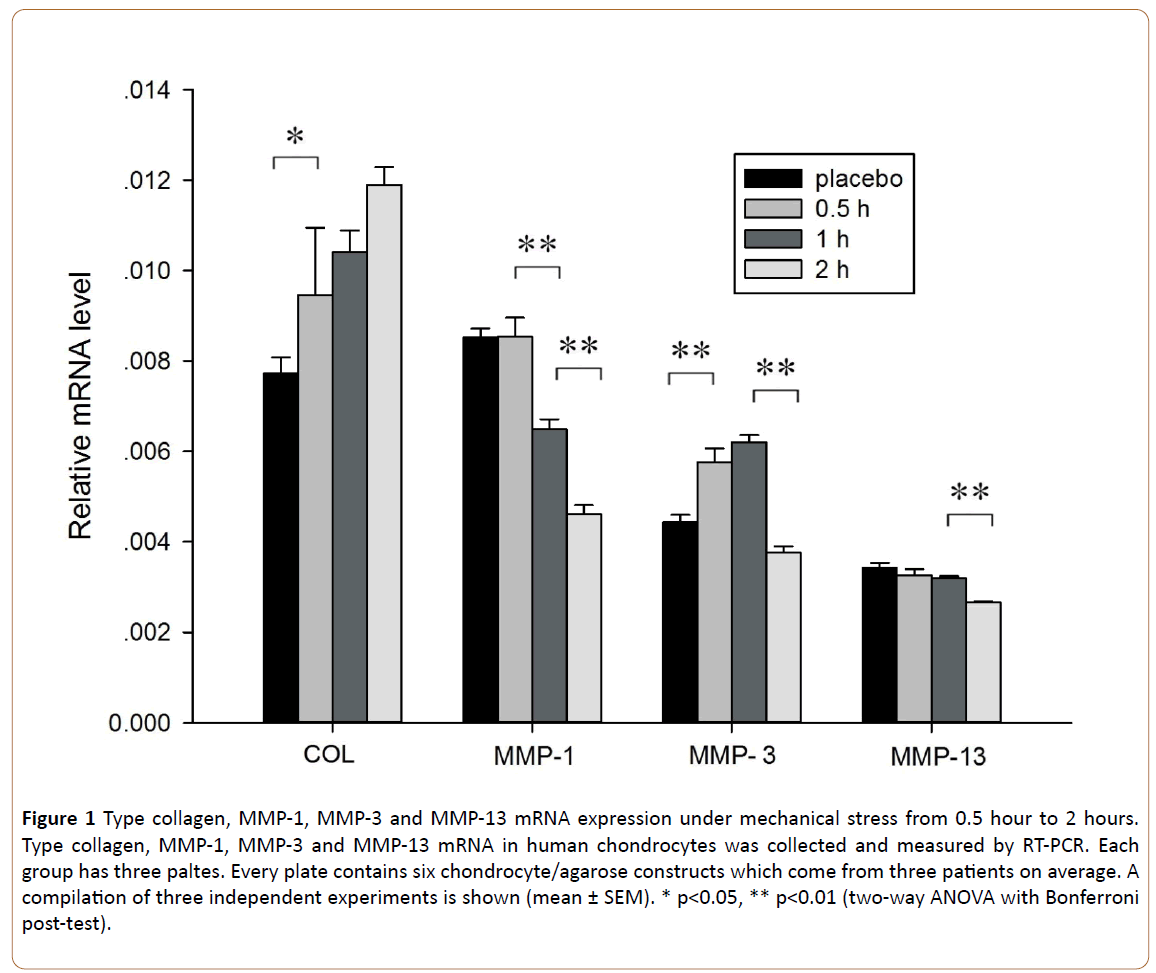
In order to investigate the effect of mechanical stress on expression of type collagen, MMP-1, MMP-3 and MMP-13 mRNA in human chondrocytes, mRNAs were collected and measured by RT-PCR. When the mechanical pressure time at 24 kPa was increased from 0 to 0.5 hour, the expression of type collagen mRNA was significantly unregulated (p=0.03; Figure 1). The expression of MMP-1 mRNA was down regulated from 0.5 hour to 2 hours (p=0.000 and 0.000 respectively; Figure 1). No significant differences in the production of MMP-13 mRNA was observed under range of pressure time from 0.5 hour to 1 hour (p=0.374, respectively; Figure 1), however MMP-13 mRNA decreased significantly in the last one hour (p=0.000; Figure 1). The trend of change in the expression of MMP-3 is inconsistent (Figure 1).
Figure 1: Type collagen, MMP-1, MMP-3 and MMP-13 mRNA expression under mechanical stress from 0.5 hour to 2 hours. Type collagen, MMP-1, MMP-3 and MMP-13 mRNA in human chondrocytes was collected and measured by RT-PCR. Each group has three paltes. Every plate contains six chondrocyte/agarose constructs which come from three patients on average. A compilation of three independent experiments is shown (mean ± SEM). * p<0.05, ** p<0.01 (two-way ANOVA with Bonferroni post-test).
The effect of mechanical stress on secretions of type collagen, MMP-1, MMP-3 and MMP-13 at protein level in chondrocytes
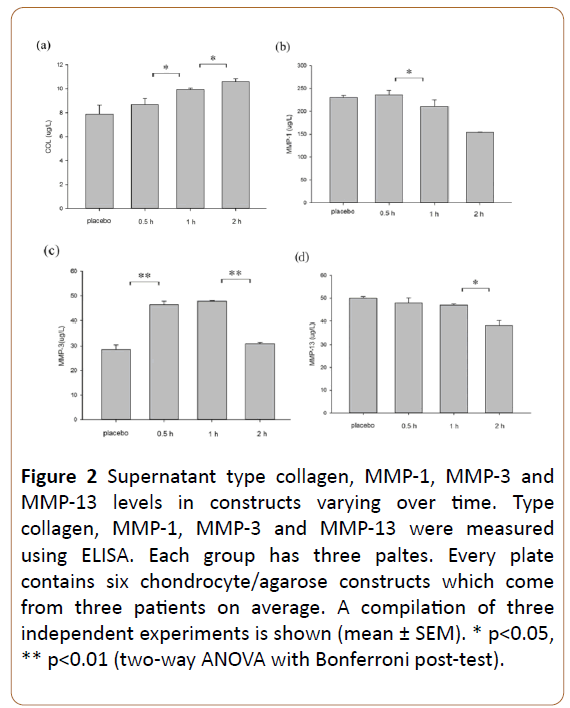
We examined if intermittent hydrostatic pressure could promote type collagen synthesis at protein level through ELISA. When the mechanical pressure time was increased from 0.5 hour to 2 hours, the expression of type collagen was significantly unregulated (p=0.016 and 0.037; Figure 2a).
The level of MMP-1 was decreased from 0.5 hour to 1 hour (p=0.017; Figure 2b).
The level of MMP-3 was decreased in the 0.5 hour, but increased later one hour, the trend of change is inconsistent (Figure 2c).
The production of MMP-13 was markedly reduced under range of pressure time from 1 hour to 2 hours (p=0.028; Figure 2d).
Figure 2: Supernatant type collagen, MMP-1, MMP-3 and MMP-13 levels in constructs varying over time. Type collagen, MMP-1, MMP-3 and MMP-13 were measured using ELISA. Each group has three paltes. Every plate contains six chondrocyte/agarose constructs which come from three patients on average. A compilation of three independent experiments is shown (mean ± SEM). * p<0.05, ** p<0.01 (two-way ANOVA with Bonferroni post-test).
Verapamil-promoted type collagen, MMP-1, MMP-3 and MMP-13 mRNA expression under mechanical stress
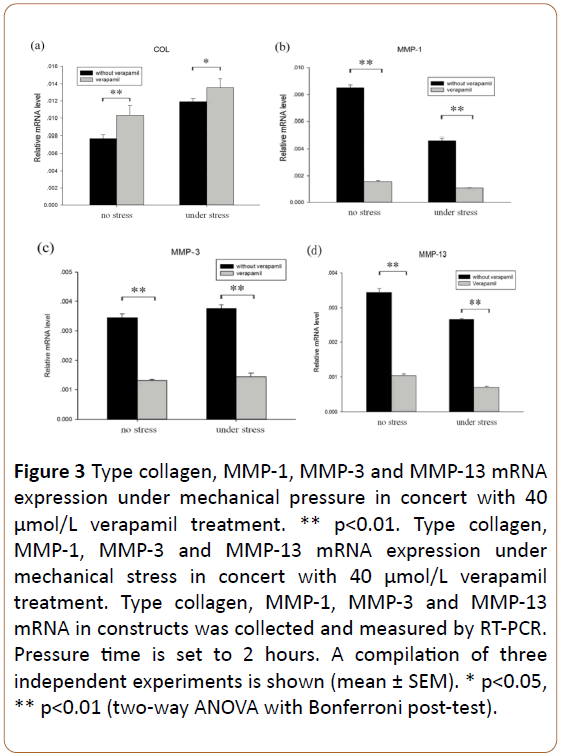
To test the potential use of verapamil as a promoter of type collagen synthesis in OA chondrocytes under mechanical pressure, we applied verapamil at the concentration of 40 μmol/L, which is a sufficient dose without cytotoxic effects to the chondrocytes [19]. Combination with or without mechanical pressure, verapamil inhibited the expression of MMP-1, MMP-3 and MMP-13 mRNA in OA chondrocytes (all p<0.01, Figures 3b-3d). Combination with or without mechanical pressure, the expression of type collagen mRNA was up-regulated in OA chondrocytes (p=0.005 and 0.037, Figure 3a).
Figure 3: Type collagen, MMP-1, MMP-3 and MMP-13 mRNA expression under mechanical pressure in concert with 40 μmol/L verapamil treatment. ** p<0.01. Type collagen, MMP-1, MMP-3 and MMP-13 mRNA expression under mechanical stress in concert with 40 μmol/L verapamil treatment. Type collagen, MMP-1, MMP-3 and MMP-13 mRNA in constructs was collected and measured by RT-PCR. Pressure time is set to 2 hours. A compilation of three independent experiments is shown (mean ± SEM). * p<0.05, ** p<0.01 (two-way ANOVA with Bonferroni post-test).
The role of verapamil in promoting type collagen synthesis can be enhanced by mechanical pressure. Verapamil combination with mechanical pressure, the expression of type collagen mRNA was raised (p=0.001, Figure 3a) while the expression of MMP-1 and MMP-13 mRNA were downregulated (p=0.004 and 0.000, Figures 3b and 3d) more obvious than verapamil alone.
Verapamil promoted type collagen expression synthesis in human osteoarthritic chondrocyte/agarose constructs
To investigate the effect of verapamil on type collagen synthesis in chondrocyte/agarose constructs under mechanical pressure.
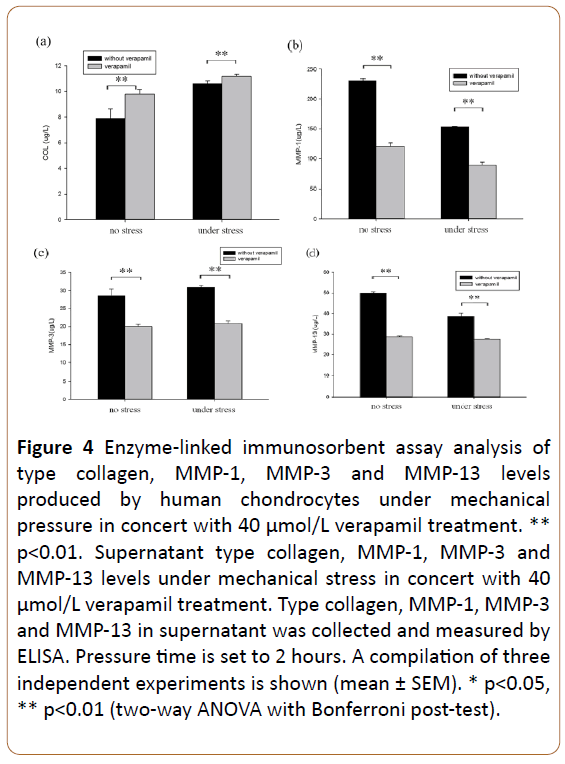
Verapamil promoted the production of type collagen (p=0.000 and 0.000; Figure 4a), and inhibited the level of MMP-1 (p=0.004 and 0.004; Figure 4b), MMP-3 (p=0.000 and 0.000; Figure 4c) and MMP-13 (p=0.005 and 0.005; Figure 4d), with or without mechanical pressure.
Figure 4: Enzyme-linked immunosorbent assay analysis of type collagen, MMP-1, MMP-3 and MMP-13 levels produced by human chondrocytes under mechanical pressure in concert with 40 μmol/L verapamil treatment. ** p<0.01. Supernatant type collagen, MMP-1, MMP-3 and MMP-13 levels under mechanical stress in concert with 40 μmol/L verapamil treatment. Type collagen, MMP-1, MMP-3 and MMP-13 in supernatant was collected and measured by ELISA. Pressure time is set to 2 hours. A compilation of three independent experiments is shown (mean ± SEM). * p<0.05, ** p<0.01 (two-way ANOVA with Bonferroni post-test).
The role of verapamil in promoting type collagen synthesis can be enhanced by mechanical pressure.
Verapamil combination with mechanical pressure, the production of type collagen was raised (p=0.000, Figure 4a) while the level of MMP-1 and MMP-13 were down-regulated (p=0.000 and 0.000, Figures 4b and 4d), more than verapamil alone affected.
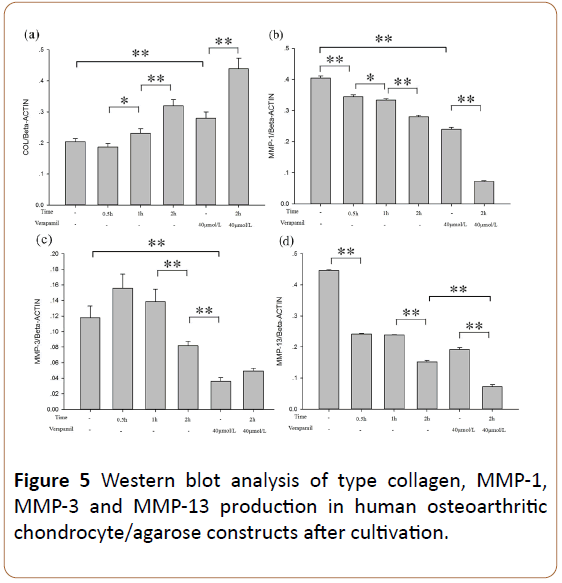
Western blot analysis of type collagen, MMP-1, MMP-3 and MMP-13 levels produced by chondrocytes
Western blot analysis of placebo and other groups revealed the metabolism of type collagen. Co-stimulation of mechanical pressure and verapamil played a catalytic role on type collagen synthesis (Figure 5). Image J software was used to measure the mean gray value of type collagen (Figure 5a), MMP-1 (Figure 5b), MMP-3 (Figure 5c) and MMP-13 (Figure 5d).
Discussion
Osteoarthritis was once considered to be a degenerative disease caused by direct cartilage injury. Recently, more and more evidence indicated that osteoarthritis was closely related to the inflammatory process in cartilage at the molecular level [20]. At present, it is widely accepted that abnormal mechanical stimulation change chondrocytes from low metabolic activity to high state [3]. PG and MMPs were released, which cause destruction of cartilage matrix, the balance between the biosynthesis and degradation of ECM is disturbed in osteoarthritis joint cartilage [21,22]. Our study investigated the influence of mechanical stress to chondrocytes biosynthesis in the chondrocyte/agarose constructs. For further explore the mechanism, verapamil was added to help biosynthesis. In this way, we confirmed that mechanical pressure resulted in increased chondrocyte type collagen biosynthesis, and verapamil promoted its influence.
Mechanical stress was considered as a regulator in chondrocyte biosynthetic activity, which is essential for maintaining the normal function of cartilage matrix. Li et al. [23] showed moderate dynamic compression (strain amplitude 10%, 20%) has an anti-catabolic role in reducing cytokinemedias cartilage degradation, while there is a threshold strain amplitude (30%), above which loading becomes detrimental to cartilage. DiMicco et al. [24] showed injurious compression of articular cartilage induces an initially high rate of proteoglycan release from the tissue, which could not be inhibited, consistent with mechanical damage. These findings suggested that pressure in different levels and frequencies have different effects on chondrocyte. Low levels of pressure can help maintain the normal function of the cartilage matrix, while long-term, high-intensity mechanical stimulation might destroy that balance [25]. In our study, we set up the pressure parameter at 24 kPa, which provides sufficient pressure stimulation for chondrocyte/agarose constructs and maintain the integrity of the three-dimensional model. The results showed a time-dependent increasing of the production type collagen while decreasing of MMP-1, MMP-13 from chondrocytes under pressure.
Ion channel is believed to be one of the possible mechanisms of mechanical pressure acting on chondrocytes. Ionomycin, a Ca2+ ionophore, can increase the intracellular calcium concentration, which increases the collagen content and tensile properties of tissue-engineered articular cartilage [26]. Recently Takamatsu et al. found verapamil could protect cartilage from degradation via inducing FRZB expression and suppress Wnt/beta-catenin signaling [16]. In our study, mechanical stress promoted type collagen synthesis in chondrocyte/agarose constructs; verapamil further strengthened the role of mechanical stress. However, Chowdhury et al. [27] found that dynamic compression (15%, 1 Hz, 48 h) can inhibits NO release, promotes the synthesis of PG, and gadolinium (a Ca2+ channel inhibitor) inhibits the effect of dynamic compression by inhibiting purinergic pathway. Therefore, it can be speculated that the role of verapamil acting on chondrocytes need to be further studied, and the mechanisms of type collagen and aggrecan may be different.
In this study, the levels of MMP-1 and MMP-13 were influenced by verapamil or by the varying durations of stress. Verapamil both independently and in combination with mechanical stress, to decrease the protein production of MMP-1, MMP-3 and MMP-13 and type collagen in human osteoarthritic chondrocytes in a three-dimensional culture. The results presented provide insight into potentially important therapeutic aspects of mechanical loading and Ca2+ channel blocking; the results suggest that both of these are possible therapeutic approaches for OA. However, the clinical effect of verapamil on blood pressure and other cardiovascular system needs to be considered as well, and the specific role needs to be further researched. The study suggested mechanical stress and Verapamil are with promoting effect on type collagen synthesis, which provided a new theoretical basis for the treatment of OA.
Conclusion
Mechanical stress, either independently or in combination with verapamil treatment, could raise the production of type collagen in human osteoarthritic chondrocytes in a threedimensional culture. This study may predict that proper physical exercise has therapeutic roles for patients with osteoarthritis and protective effect for articular cartilage in healthy population.
Authors Contributions
Guan Jian-long helped to design the study, performed the immunohistochemistry, analyzed the data and modified the manuscript. Luo Dan and Shen Yan carried out the cell isolation and culture, the preparation of chondrocyte/agarose constructs, compression testing, performed real-time PCR and Western blot analysis, and drafted the manuscript. LV Jian- Qiang participated in the preparation of chondrocyte/agarose constructs, compression testing. Fan Yong-Qian, Huang Dong- Hui, Lin Wei-Long, Shen Hei-Min participated in the cell isolation and immunohistochemistry. Xu Hu-Ji participated in the design of the study and performed the statistical analysis. All authors read and approved the final manuscript.
Acknowledgements
This work was funded by National Natural Science Foundation of China, project No. 81072478, 81273298. We also give great thanks to other colleagues in our department, Zou Jun, Cai Jian-Fei, He Pei-Qing, Chen Yong, Bao Hua-Fang, YE Jing-Fen for their support and help in this research.
References
- Brooks PM (2002) Impact of osteoarthritis on individuals and society: how much disability? Social consequences and health economic implications. Curr Opin Rheumatol 14: 573-577.
- Fautrel B, Hilliquin P, Rozenberg S, Allaert FA, Coste P, et al. (2005) Impact of osteoarthritis: results of a nationwide survey of 10,000 patients consulting for OA. Joint Bone Spine 72: 235-240.
- Loeser RF (2006) Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide[J]. Arthritis Rheum 54: 1357-1360.
- Responte DJ, Natoli RM, Athanasiou KA (2007) Collagens of articular cartilage: structure, function, and importance in tissue engineering. Crit Rev Biomed Eng 35: 363-411.
- Bruckner P, van der Rest M (1994) Structure and function of cartilage collagens. Microsc Res Tech 28: 378-384.
- Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11: 224.
- Tetlow LC, Adlam DJ, Woolley DE (2001) Matrix metalloproteinase and proinflammatory cytokine production by chondrocytes of human osteoarthritic cartilage: associations with degenerative changes. Arthritis Rheum 44: 585-594.
- Elliott S, Hays E, Mayor M, Sporn M, Vincenti M (2003) The triterpenoid CDDO inhibits expression of matrix metalloproteinase-1, matrix metalloproteinase-13 and Bcl-3 in primary human chondrocytes. Arthritis Res Ther 5: R285-R291.
- Tong KM, Chen CP, Huang KC, Shieh DC, Cheng HC, et al. (2011) Adiponectin increases MMP-3 expression in human chondrocytes through AdipoR1 signaling pathway. J Cell Biochem 112: 1431-1440.
- Freije JM, Díez-Itza I, Balbín M, Sánchez LM, Blasco R, et al. (1994) Molecular cloning and expression of collagenase-3, a novel human matrix metalloproteinase produced by breast carcinomas. J Biol Chem 269: 16766-16773.
- Griffin TM, Guilak F (2005) The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev 33: 195-200.
- Millward-Sadler SJ, Wright MO, Flatman PW, Salter DM (2004) ATP in the mechanotransduction pathway of normal human chondrocytes[J]. Biorheology 41: 567-575.
- Mellstrom B, Naranjo JR (2001) Mechanisms of Ca2+-dependent transcription. Curr Opin Neurobiol 11: 312-319.
- Pingguan-Murphy B, Lee DA, Bader DL, Knight MM (2005) Activation of chondrocytes calcium signalling by dynamic compression is independent of number of cycles. Arch Biochem Biophys 444: 45-51.
- Spedding M, Paoletti R (1992) Classification of calcium channels and the sites of action of drugs modifying channel function. Pharmacol Rev 44:363-376.
- Takamatsu A, Ohkawara B, Ito M, Masuda A, Sakai T (2014) Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/beta-catenin signaling. PLoS One 9: e92699.
- Altman R, Asch E, Bloch D, Bole G, Borenstein D et al. (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29: 1039-1049.
- Bougault C1, Paumier A, Aubert-Foucher E, Mallein-Gerin F (2009) Investigating conversion of mechanical force into biochemical signaling in three-dimensional chondrocyte cultures. Nat Protoc 4: 928-938.
- Xu J, Wang W, Clark CC, Brighton CT (2009) Signal transduction in electrically stimulated articular chondrocytes involves translocation of extracellular calcium through voltage-gated channels. Osteoarthritis Cartilage 17: 397-405.
- Attur MG, Dave M, Akamatsu M, Katoh M, Amin AR (2002) Osteoarthritis or osteoarthrosis: the definition of inflammation becomes a semantic issue in the genomic era of molecular medicine. Osteoarthritis Cartilage 10: 1-4.
- Loeser RF (2006) Molecular mechanisms of cartilage destruction: mechanics, inflammatory mediators, and aging collide. Arthritis Rheum 54: 1357-1360.
- Goldring MB, Marcu KB (2009) Cartilage homeostasis in health and rheumatic diseases. Arthritis Res Ther 11: 224.
- Li Y, Frank EH,Wang Y,Chubinskaya S, Huang Han-Hwa (2013) Moderate dynamic compression inhibits pro-catabolic response of cartilage to mechanical injury, tumor necrosis factor-alpha and interleukin-6, but accentuates degradation above a strain threshold. Osteoarthritis Cartilage 21: 1933-1941.
- DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA et al. (2004) Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum 50: 840-848.
- Buckwalter JA, Martin JA, Brown TD (2006) Perspectives on chondrocyte mechanobiology and osteoarthritis. Biorheology 43: 603-609.
- Natoli RM, Skaalure S, Bijlani S, Chen KX, Hu J, et al. (2010) Intracellular Na(+) and Ca(2+) modulation increases the tensile properties of developing engineered articular cartilage. Arthritis Rheum 62: 1097-1107.
- Chowdhury TT, Knight MM (2006) Purinergic pathway suppresses the release of .NO and stimulates proteoglycan synthesis in chondrocyte/agarose constructs subjected to dynamic compression. J Cell Physiol 209: 845-853.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences