ISSN : 2634-7806
Journal of Clinical and Molecular Pathology : Open Access
Prevalence, Types and Antibiotic Sensitivity Pattern in Urinary Tract Infection (UTI) In Midnapore Town, India
Aarifa Nazmeen and Smarajit Maiti*
Dept. of Biochemistry, Cell & Molecular Therapeutics Lab, Oriental Institute of Science & Technology, Midnapore, 721101, India.
- *Corresponding Author:
- Smarajit Maiti
Associate Professor and Head Post Graduate, Department of Biochemistry and Biotechnology, Cell & Molecular Therapeutics Lab, OIST, Midnapore-721102
Tel: 947450426
E-Mail: maitism@rediffmail.com
Received date: January 1, 2018; Accepted date: June 11, 2018; Published date: June 15, 2018
Citation: Maiti S, Nazmeen A (2018) Prevalence, Types and Antibiotic Sensitivity Pattern in Urinary Tract Infection (UTI) In Midnapore Town, India. Journal of Clinical and molecular Pathology Vol 2:16.
Copyright: © 2018 Maiti S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Urinary-tract-infection (UTI) is a major health/hygiene concern in the community with highest morbidity due to the fact of increasing multidrug-resistance in bacterial-uropathogens. Objective: Studies on prevalence of UTI in semi-urban Indian localities. Pattern study of antibiotic-resistance was investigated found in UTI. Aim was also to study the discrepancies in the sensitivity and resistant pattern of different pathogens against particular drug. Material & Methodology: Urine sample were collected (19-males/33-females) from Midnapore scan-center, West Bengal and analyzed. Result: The 40% of the UTI is attributed by E.coli followed by K. oxytocae (17%), Staphylococcus aureus (14%) (S.aureus). Female to male infection was 63:37(Figure-1a). The drug susceptibility response suggests that 70-100% of the bacterial isolates from the patients were susceptible to 26% of the drugs in case of E. coli, 36.66% in case of Klebsiella sp. and Enterrococci sp., 50% in case of Citrobacter sp. 53.33% of the drugs in case of S.aureus and 73.33% in case of Proteus sp. Conclusion: E. coli is resistant to most of the drugs. Drug-sensitivity pattern of individuals suggests that when a larger number of drugs behave sensitively against Citrobactor sp. in more number of patients, a small number of drugs are sensitive to S. aureus in in more number of patients.
Keywords
Urinary tract infection; Antibiotic resistance; Drug-sensitivity pattern; India
Abbreviation
MR: Meropenem; CAL: Clavulanic acid; AT: Aztreonam; DO: Doxycycline; OF: Ofloxacin; CA: Ceftazidime; CO: Colistin; PR: Para-aminosalicylic acid; CTX: Cefotaxime; NX: Norfloxacin; CTR: Ceftriaxone; CFM:Cefixime; LE: Levofloxacin; AK: Amikacin; PT: Piperacillin-tazobactam; CPZ: Cefprozil; CXM: Cefuroxime; GM: Gentamicin; NA: Nalidixic Acid; AMC: Amoxycillin; AZM: Azithromycin; COT: Trimethoprim-sulfamethoxazole; GEN: Gentamicin; CAC: Cefaclor; GEM: Gemifloxacin; PRU: Ulifloxacin; NIT: Nitrofurantoin; TB: Tobramycin; CAZ: Ceftazidime.
Introduction
Urinary tract infection (UTI) is a major public health problem and the commonest bacterial infectious disease in the community with a high rate of morbidity. An estimated 150 million people were infected with UTI per annum worldwide which may cost a global economy more than 6 billion US dollars [1]. Clinical studies suggest that the overall prevalence of UTI is higher in women; less complicated UTIs in healthy women have an incidence of 50/1000/year. UTI varies with age and gender, boys between the ages of 1-5 year suffers UTI more frequently and need to be evaluate efficiently [2]. A study conducted to determine the prevalence of community acquired-UTI in rural Odisha showed that prevalence of UTI in females was 45.2% [3]. The lower UTI is defined by the term cystitis and is characterized by symptoms such as dysuria, frequency, urgency, and suprapubic tenderness. The infection usually begins from the lower urinary tract and spreads along upper UTI which is often present in most UTI cases [4]. UTI can be classified into uncomplicated and complicated on the basis of their choice of treatment [5]. UTI is more common in females than in males as female urethra structurally found less effective for preventing the bacterial entry [6], due to the closeness of the genital tract and urethra [7], adherence of urothelial mucosa to the mucopolysaccharide lining [8], menstrual unhygienic practices [9] and using birth control diaphragm [10]. Possibility is that Menstruation and its hygiene management creates abnormally moist conditions in the urogenital that may promote bacterial invasion. The other main factors which make females more prone to UTI are pregnancy and sexual activity [11]. In pregnancy, physiological plasma volume increases and decrease in urine concentration develop glycosuria in up to 70% women which ultimately leads to bacterial growth in urine [12]. Urinary catheter-related infection leads to substantial morbidity and mortality. The incidence of bacteriuria in catheterized patients varies between 3% and 10% per day [13]. A large spectrum of bacteria causes complicated UTI and a number of bacteria cause uncomplicated UTI. However, the most commonly encountered microorganisms are Gram negative bacteria including Escherichia coli, Citrobacter spp., Enterobacter aerogenes, Enterococci sp., Pseudomonas aeruginosa and Proteus sp. whereas Klebsiella spp., Staphylococcus aureus and Salmonella spp. are found rarely [14]. UTIs are treated with empirical antimicrobial. Increasing multidrug resistance in bacterial uropathogens is an important and emerging public health problem. Some microorganisms are identified as “ESKAPE pathogens” by the Infectious Disease Society of America (IDSA) which needs new effective therapies. Those microorganisms include Enterococcus faecium, S. aureus, Klebsiella sp., Acinetobacter spp., Pseudomonas spp., and Enterobacter spp.
Uropathogenic bacteria are now evolving as multi drug-resistant, showing resistance to more than 2 antibiotics, this is an alarming situation and this leaves the treating physician with few choices of antimicrobial agents to treat UTI. There are more than 15 classes of antibiotics whose targets are involved in essential physiological or metabolic functions of the bacterial cell [15]. Drug resistance genes are one of the causes. The genes for resistance traits can be transferred among bacteria of different taxonomic and ecological groups by means genetic elements such as bacteriophages, plasmids, naked DNA or transposons [15,16]. These genes are generally directed against a single family or type of antibiotic, multiple such genes, each bearing a single drug resistance trait, can accumulate in the same organism. Eventually resistance genes and their hosts spread and propagate to amplify and extend the problem to other hosts and other geographic locations. Low-level to high-level resistance occurs in bacteria through sequential mutations in chromosomes [15,17,18]. E.coli and other Enterobacteriaceae strains have evolved increasing resistance to fluoroquinolones for mutations in the target enzymes (topoisomerases) and increment of membrane proteins that pump the drugs out of the cell [17,18,19]. Genetic analyses suggest that the long-anticipated transfer of vancomycin resistance to a methicillin-resistant S. aureus occurred in vivo by interspecies transfer of Tn1546 from a co-isolate of Enterococcus faecalis [20]. Biofilms are the predominant phenotype of nearly all the bacteria in their natural habitats, whether they are pathogenic or environmental. Bacterial pathogens form biofilms which protect them from starvation, desiccation and antibiotics. Multidrug resistant strains are efficient biofilm producers, indicating a direct relationship between biofilm formation and antibiotic resistance [21]. E.coli pathotypes reside harmlessly in the human intestinal microenvironment but, upon access to sites outside of the intestine, become a major cause of human morbidity and mortality as a consequence of invasive UTI (pyelonephritis, bacteremia, or septicaemia [22]. E.coli can form biofilms in the bladder during a urinary tract infection, extracellular matrix (ECM), of E. coli is primarily composed of curli a protein polymer and the polysaccharide cellulose promoting adherence to organic and inorganic surfaces and resistance to desiccation, the host immune system, and other antimicrobials [23]. The biofilm formation of urinary S.aureus strains was low. The strains showed higher resistance to beta-lactams, in other cases resistance was low [24].
UTI needs a regular monitoring of the antibiotic susceptibility of uropathogens in any particular area because of increasing drug resistance. Factors such as the type of UTI (complicated or uncomplicated), gender, age, and previous history of antibiotic therapy of each UTI patient should also be considered to find out the correct global data on susceptibility and for further appropriate treatments attempts [25]. Antimicrobial susceptibility data of UTI-causing microorganisms changes from time to time and from place to place [26]. Data provided by regional microbiology laboratories on the susceptibility patterns helps to choose the empirical choice of antimicrobials to treat UTI [27,28]. Generally, the antimicrobial treatment is initiated before the laboratories results which may lead to the frequent misuse of antibiotics [29]. The resistance pattern of community acquired uropathogens has not been extensively studied in India [30-32]. Different antimicrobial agents act in different ways. The understanding of these mechanisms as well as the chemical nature of the antimicrobial agents is crucial in the understanding of the ways how resistance against them develops. Broadly, antimicrobial agents may be described as either bacteriostatic or bactericidal. This increased antimicrobial resistance which is the result of irrational and uncontrolled use of the antimicrobials is threat to the public health. The aim of the study is to determine the prevalence of UTI in male and female patients as well as the effect of gender and age on its prevalence. The UTI-causing microorganisms, their distribution among different ages and genders were studied in the current investigation. Other objective was to assess the current antibiotic resistance pattern in the common uropathogens isolated in a tertiary care hospital Midnapore, West Bengal, India.
Materials and Methods
Study area
The study was carried out in Midnapore scan center, Medinipore district, West Bengal.
Sample size
The urine samples of 52 patients comprised of 19 males and 33 females, who had clinical evidence of urinary tract infection as suggested by the physician, were included in this study. The age of the patients included in the study ranged from <1 to 77 years. Patients with hospital acquired infections and patients on antibiotic therapy were excluded from the study.
Sample collection
The study was conducted after the ethical approval which was subjected to the hospital administrations. All patients were carefully instructed on how to collect sample aseptically to avoid external contaminations. In each container boric acid (0.2 mg) was added to prevent the growth of bacteria in urine samples.
Sample processing
Plating, culture and Isolation of the bacterial pathogens were conducted by eligibly experienced and authorized lab technicians in a highly aseptic laboratory condition. All the microscopic examination involving identification of bacterial strains was also performed by authorized lab technicians.
Identification of gram positive and gram negative bacteria
Gram negative and positive bacteria were identified by the standard Gram staining method using Crystal violet, iodine and safranin.
Identification by biochemical characteristics
The bacterial species where structurally identified on the basis of Gram staining including shape, motility, capsule, spore, flagella. Isolated cultures were biochemically identified for catalase, MR, VP, OF (Oxidative/ Fermentative), Indole, Citrate, Urease, nitrate reduction. Then for the third level of identification isolated cultures were subjected to fermentation of Arabinose, DNase, Fructose, Glucose, Inositol, Lactose, Maltose, Sorbitol and sucrose. Isolated cultures were also examined for certain enzymatic reaction such as Acetate utilization, acid phosphatase, alkaline phosphatase, amidase, lysine, ONPG test and phenylalanine deaminase.
Bacteria culture
The isolates were subculture periodically according to the requirement in the following media.
E.coli- Macconkey agar media, S.aureus- Mannitol salt agar (MSA) media,
Citrobacter sp.- Eosin methylene blue agar media (EMB), K.oxytocae- Nutrient agar media, Enterrococci sp.- Bile esculin agar (BEA) media, Proteus sp.- Levine EMB agar media
Antibiotic selection and antimicrobial disc susceptibility test
30 antibiotics were selected for the study and antimicrobial disc susceptibility test was performed following the standard kit method (Himedia).
Results
Prevalence rate and frequency distribution of UTI among different age groups
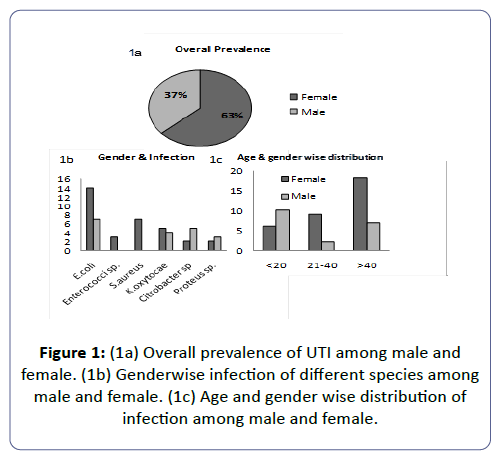
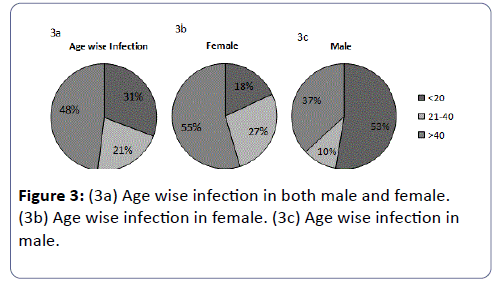
The total prevalence of UTI in female patients was found to be 63% and in males about 37%. These results indicated that the prevalence of UTI was higher in female patients than in males. The highest susceptible age group of UTI patients irrespective of gender was found to be >40 years (48%) followed by ≤ 20 years (31%), and then between 21-40 years (21%).
Some variations were found in age wise susceptibility between males and females. The highest prevalence of UTI in females was found in the age group of >40years (55%); followed by an age group between 21-40 (27%) years and the least susceptible age group was ≤ 20 years (18%), however in males the highest susceptible age group to UTI was ≤ 20 years (53%), followed by age greater than 40 years (37%) and then the least susceptible group was between 21-40 years. Eventually it was found that females are markedly more susceptible to UTI in comparison to males.
Distribution frequency of isolated bacterial uropathogens
Among all the isolated bacterial uropathogens from UTI patients, Escherichia coli was found as the dominant bacteria with the prevalence rate of 40%. The second most prevalent isolate was Klebsiella oxytocae (17%) followed by Staphylococcus aureus (14%), Citrobacter sp. (13% Proteus sp. (10%) and Enterococci sp. (6%).
Gender-wise distribution of uropathogens
The prevalence rate for the occurrence of different uropathogens among males and females were as follows, E. coli infection in females was higher being (43%) than in males (37%). Infection by Enterrococci sp. was found only in females comprising 9 of the UTI’s conversely being 0 in males. S. aureus comprised 21 of the females UTI and 0 of male UTI’s. K. oxytocae caused 21 of the male UTI’s and 15 of female UTI’s. Citrobactersp. caused 26% of male UTI’s whereas only 6 of the female UTI’s. Proteus sp.covered 16 of the male UTI’s and only 6 of the female UTI.
Drug resistance
E. coli against tested antibiotics: CAL, NF, NIT was found to be sensitive against 100% of the E. coli infected individuals followed by MR, AK, G having ≥ 90%sensitivity, following AT, PT and CAC sensitive against ≤ 70% where AT, PT was Intermediate for 30 and CAC for 10 and resistant to the remaining. ≥ 50 of the E.coli infected individuals was sensitive to CA, CTX, CTR (40% Intermediate, 10 Resistant), GM, AZM and GEN (20 Intermediate). The least sensitive and highly resistant drugs were DO, OF, CO, AC, CPZ, TB, CAZ, PR (<50 sensitive), CXM, AMC, COT and GEM (10 sensitive).
NA and PRU were resistant to 90% of the E. coli infected individuals and intermediate to the remaining 10%.
K. oxytocae against tested antibiotics: NF, CPZ, GEN and NET was found to be sensitive for 100% of the Klebsiella infected individuals. CAL, AK, PT, CXM was sensitive against >70% of the infected individuals and resistant to the remaining. MR, AT, OF, CA, CTX, NX, LE (15% I), G, AZM (50% I) and COT were sensitive to ≥ 50% of the infected individuals. DO, PR (10% I), CTR (15% I),
CFM (10% I) were sensitive to <50% of the infected individuals and resistant to the remaining. CO, AC, GEM, NA, GM, PO were resistant to 100% of the infected individuals and AMC was resistant to 50% and intermediate for the 50% of the infected individuals.
Enterrococci sp. against tested antibiotics: CAL, LZ, VA, PT, AC, CXM, CAC, CTX and NF were sensitive to 100% of the infected individuals. MR (<30% I) and G (30% R) were sensitive to ≥ 70% of the infected individuals. CX (<50% R) and OX (<50% I) were sensitive to ≥ 50% of the infected individuals. L, CFM and AK were sensitive to <50% of the infected individuals and resistant for the remaining. OF was resistant to >60% of the infected individuals and intermediate for the remaining.
AT, DO, SC, LE, GEM, PR, SPX, NX, CS were resistant to 100% of the infected individuals.
S. aureus against tested antibiotics: L, LL, VA, CAL, AK, PT, G, LZ, NF, GEN, CXM were sensitive to 100% of the infected individuals.MR (15% R), DO (35% R), CX (15% I & 15% R), CAC (20% R) were sensitive to >60 but <100% of the infected individuals. LE (>30% I, 30% R), AC (15% S, 85% R), PR (20% S, 25% I, 55% R) and GM (70% I) were sensitive to <50% of the individuals. NX was intermediate for 100% of the infected individuals. OF, SPX, MO was resistant to 50% and intermediate to the 50% of the infected individuals.
AT, CTX, CPZ, AMC and AZM were resistant to 100% of the infected individuals.
Citrobacter sp. against tested antibodies: PT, TB, MR, CAC, AK, GRN, AZM, NET, LE, CAL, AT were sensitive to 100% of the Citrobacter infected individuals. COT, CTR, CFM, CAZ, CTX, PRU, G, CA were sensitive to ≥ 50% of the infected individuals and resistant to less than 50% of the infected individuals.
PR, NX, CXM, CO, PRV, DO were sensitive to <50% infected individuals and resistant to >50% of the individuals. AMC was resistant to 50% and intermediate to the 50% of the infected individuals.
NA, CPZ, GEM, GM were resistant to (bacterial isolation of) 100% of the infected individuals.
Proteus sp. against tested antibiotics: MR, CAL, OF, CA, CO, PR, CTX, NX, CTR, CFM, LE, AK, PT, G, CAC, CPZ, COT, TB, CAZ, AMC, GEN and PRV were sensitive to the 100% of the infected individuals.
DO, GM, NF, AZM were resistant to 100% of the infected individuals. AL was intermediate to the 100% of the infected individuals. AC was resistant to >50% and sensitive to <50% of the infected individuals.
Discussion
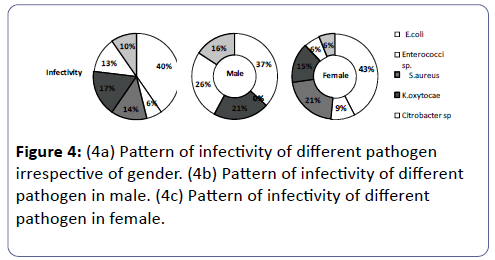
This study may provide a valuable information on the present scenario of UTI infection and antimicrobial resistance/sensitivity pattern in the district of Midnapore (WB) to improve efficient empirical treatment. Increasing antimicrobial resistance has been documented globally [33-39]. The prevalence of UTI was found to be high including various uropathogens such as E. coli, Enterrococci sp., S. aureus, Klebsiella sp. citrobacter sp. and Proteus sp. Highest abundance and attribution of E. coli (40%) in UTI has been noticed in our studies followed by K.oxytocae (17 %), S.aureus (14%), Citrobacter sp.(13%), Proteus sp. (10%) and Enterrococci sp.(6%),The proportionate contribution of the first three bacteria’s for the infection is like 40%:17%:14% including both male and in the female (Figure 4a). This result is consistent with reports from other studies [3,40-45] but differs from the reports in which P. aeruginosa [46] and Klebsiella sp. [47] were recorded as the predominant bacteria in UTI. however, these results correlates with others in which Klebsiella spp. was reported as the second most frequently isolated organism in UTI [48-52]. The studies on UTI in other places of the world also showed that E. coli and Klebsiella spp. are the commonest uropathogens in UTI [31,32,53-55]. A gender associated difference in the infection was noticed, ratio of the percentage of male to female infection was 63:37 being in accordance with earlier studies (Figure 1a) [7,56-61].
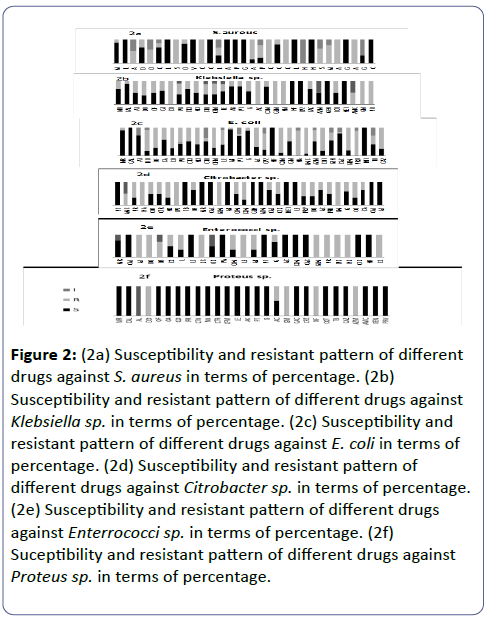
Figure 2: (2a) Susceptibility and resistant pattern of different drugs against S. aureus in terms of percentage. (2b) Susceptibility and resistant pattern of different drugs against Klebsiella sp. in terms of percentage. (2c) Susceptibility and resistant pattern of different drugs against E. coli in terms of percentage. (2d) Susceptibility and resistant pattern of different drugs against Citrobacter sp. in terms of percentage. (2e) Susceptibility and resistant pattern of different drugs against Enterrococci sp. in terms of percentage. (2f) Suceptibility and resistant pattern of different drugs against Proteus sp. in terms of percentage.
E. coli was the predominant in both the genders comprising of 43% in female (Figure 3 and Figure 4c) and 37% in males (Figure 4b). In females of all age categories, E. coli is the most frequently isolated uropathogen which correlates with other studies [62-64] in contradiction to somewhere E. coli causes most male UTIs, followed by other Enterobacteriaceae and Enterococci [65,66] second predominant bacteria in males was found to be Citrobacter sp. (26%) (Figure 4b) whereas only 6% in females (Figure 4c), 21% of males (Figure 4b) and 15% females were infected by Klebsiella sp. (Figure 4c), 16% of the males (Figure 4b) and 6% of females (Figure 4c) were infected by Proteus sp., 21% of females (Figure 4c) were infected by S. aureus and 9% of females (Figure 4c) were infected by Enterrococci sp. and both had no participation in male infection. It seems like pathogens like Citrobacter sp. and Proteus sp. are more specific to males whereas Proteus sp. are specifically involved in female infection.
Selected antimicrobials included in this study possessed both resistance and sensitivity patterns among different uropathogens. Among all the drugs included, NF showed 100% sensitivity against uropathogens like E.coli, Enterrococci sp., S. aureus, Klebsiella sp. all the pateints infected with these four bacteria showed response to NF. 100% of the individuals carrying E.coli infection responded to NIT, CAL and NF (Figure 2c), 100% of the individuals carrying Enterrococci infection responded to CAL, AC, LZ, CXM, CAC, NF, CTX, 100% of those carrying Klebsiella sp infection responded to NF, CPZ, GEN, NET (Figure 2b), 100% of Citrobacter sp. infected individuals showed response to PT, TB, MR, CAC, AK, GEN, AZM, CAL, AT (Figure 2d). 100% of Proteus sp.infected individuals responded positively to MR,CAL. OF, CA, CO, PR, CTX, NX, CTR, CFM, LE, AK, PT, G, CAC, CPZ, COT, TB, CAZ, AMC, GEN, PRV, though Proteus infection occurs least (Figure 2f). E.coli showed 100% resistance to NA, PRU, CXM, AMC (Figure 2c), Enterrococci sp.was resistant to SPX and NX (Figure 2e), S.aureus possessed resistance against AMC, SC, CPZ, CFM (Figure 2a). Klebsiella sp. possessed resistance against CO, GM, PO, AC, GE, NA. Some correlations exist between antibiotic resistance and the biofilm-forming ability of K.pneumonia strains [32]. Citrobacter sp. were resistant to CPZ, GM, NA, AZM (Figure 2d). Proteus sp.possessed resistance against DO, GM, NF, AZM (Figure 2f).
The drug susceptibility response suggests that 70-100% of the bacterial isolates from the patients were susceptible to 26% of the drugs in case of E. coli, 36.66% in case of Klebsiella sp. and Enterrococci sp., 50% in case of Citrobacter sp. 53.33% of the drugs in case of S. aureus sp. and 73.33% in case of Proteus sp. The greater extent of E.coli resistant to most of the drug increases its occurrence and recurrence which is evident from the Pie chart. This is possibly due to the intrinsic and extrinsic support of interactive environmental factors that favors the metabolic and survival benefit to the organism in the natural condition. Since E.coli resides harmlessly in the human intestine and tolerates various antibiotics being consumed by humans cause of various diseases, and when this E. coli access the urinary tract becomes a major cause of infection with increased resistance due to previous inappropriate use of antimicrobial agents. The 13% patients are found to be infected by each S. aureus and Citrobacter sp. suggesting their parity in their infectivity and propagation. But, their drug sensitivity pattern in number (%) of individuals suggests that when a larger number of drugs behave sensitively against Citrobacter sp. in more number of patients a small number of drugs are sensitive to S. aureus in more number of patients. The result shows that S. aureus is more resistant than Citrobacter sp. against a large number of drugs. Possibly, as explained earlier, the infectivity/ propagation in individuals and the sensitivity/ resistance in vivo or in vitro may not coincide due to some environmental or intrinsic/extrinsic factors. Further investigation is necessary to conclude definitive.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interests with respect to the authorship and/or publication of this article.
Acknowledgement
University Grants Commission, New Delhi provided JRF and SRF to AN who is a Ph.D. students working in the Post Graduate Department of Biochemistry, OIST.
References
- Gonzalez CM, Schaeffer AJ (1999) Treatment of urinary tract infection: what’s old, what’s new, and what works. World J Urol 17: 372-382.
- Gupta P, Mandal J, Krishnamurthy S, Barathi D, Pandit N (2015) Profile of urinary tract infections in paediatric patients. Indian Med Res 141: 473-477.
- Dash M, Padhi S, Mohanty I, Panda P, Parida B (2013) Antimicrobial resistance in pathogens causing urinary tract infections in a rural community of Odisha, India. J Family Community Med 20: 20-26.
- Sobel JD, Kaye D (2010) Urinary tract infections. Philadelphia, USA.
- Sabra SM, Abdel-Fattah MM (2012 ) Epidemiological and microbiological profile of nosocomial infection in Taif hospitals, KSA (2010-2011). World J Med Sci 7: 1-9.
- Warren JW, Abrutyn E, Richard Hebel J, Johnson JR, Schaeffer AJ, et al. (1999) Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Clin Infect Dis 29: 745-758.
- Schaeffer AJ, Rajan N, Cao Q, Anderson BE, Pruden DL, et al. (2001) Host pathogenesis in urinary tract infections. Int J Antimicrob Agents 17: 245-251.
- Akortha EE, Ibadin OK (2008) Incidence and antibiotic susceptibility pattern of Staphylococcus aureus amongst patients with urinary tract infection (UTI) in UBTH Benin City, Nigeria. Afr J Biotechnol 7: 1637-1640.
- Sumpter C1, Torondel B (2013) A systematic review of the health and social effects of menstrual hygiene management. PLoS One: 8
- Okonko IO, Ijandipe LA, Ilusanya OA, Ejembi J, Udeze AO,et al. (2009) Incidence of urinary tract infection (UTI) among pregnant women in Ibadan, South-Western Nigeria. Afr J Biotechnol 8: 6649-6657.
- Arul KC, Prakasam KG, Kumar D, Vijayan M (2012) A cross sectional study on distribution of urinary tract infection and their antibiotic utilization pattern in Kerala. International Journal of PharmTech Research 3: 1309-1316.
- Lucas MJ, Cunningham FG (1994) Urinary infection in pregnancy. Clinical Obstetrics and Gynecology. 36: 855-868.
- Sanjay Saint, Carol E. Chenoweth (2003) Biofilms and catheter-associated urinary tract infections. Infect Dis Clin North Am 17: 411-432
- Foxman B, Brown P (2003) Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am 17: 227-241.
- Levy S (2002) The Antibiotic Paradox: How Misuse of Antibiotics Destroys their Curative Powers (Perseus Cambridge, 2002). 37 pp
- Levy S Schneiders T.B. & Miller, R.V. (eds.) Gene Transfer in the Environment (McGraw Hill, New York, 1989).
- Schneiders T, Amyes SG, Levy SB (2003) Role of AcrR and ramA in fluoroquinolone resistance in clinical Klebsiella pneumoniae isolates from Singapore. Antimicrob Agents Chemoth er 47: 2831-2837.
- Wang H, Dzink-Fox JL, Chen M, Levy SB (2001) Genetic characterization of highly fluoroquinolone-resistant clinical Escherichia coli strains from China: role of acrR mutations. Antimicrob Agents Chemother 45: 1515-1521.
- Piddock LJ (1999) Mechanisms of fluoroquinolone resistance: an update 1994-1998. Drugs Suppl 2: 11-18.
- Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, et al. (2003) Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus. Science 302: 1569-71.
- Hufnagel DA, Depas WH, Chapman MR (2015) The Biology of the Escherichia coli Extracellular Matrix. Microbiol Spectr vol 3.
- Alteri CJ, Mobley HL (2015) Metabolism and Fitness of Urinary Tract Pathogens. Microbiol Spectr vol 3.
- Hufnagel DA, Depas WH, Chapman MR (2015) The Biology of the Escherichia coli Extracellular Matrix. Microbiol Spectr vol 3.
- Cernohorska L (2010) Antibiotic resistance and biofilm formation in Staphylococcus aureus strains isolated from urine. Klin Mikrobiol Infekc Lek.16: 196-198.
- Alos JI (2005) Epidemiology and etiology of urinary tract infections in the community. Antimicrobial susceptibility of the main pathogens and clinical significance of resistance. Enfermedades Infecciosas Microbiologia Clinica 4: 3-8.
- Okonko IO, Ijandipe LA, Ilusanya OA, Ejembi J, Udeze AO, et al. (2009) Incidence of urinary tract infection (UTI) among pregnant women in Ibadan, South-Western Nigeria. Afr J Biotechnol 8: 6649-6657.
- McNulty CAM, Richards J, Livermore DM (2006) Clinical relevance of laboratory-reported antibiotic resistance in acute uncomplicated urinary tract infection in primary care. J Antimicrob Chemother 58: 1000-1008.
- Car J (2006) Urinary tract infections in women: diagnosis and management in primary care. British Medical Journal 332: 94-97.
- Tambekar DH, Dhanorkar DV, Gulhane SR, Khandelwal VK, Dudhane MN, et al. (2006) Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol 5: 1562-1565.
- Biswas D, Gupta P, Prasad R, Singh V, Arya, et al. (2006) Choice of antibiotic for empirical therapy of acute cystitis in a setting of high antimicrobial resistance. Indian J Med Sci 60: 53-58.
- Akram M, Shahid M, Khan AU (2007) Etiology and antibiotic resistance patterns of community-acquired urinary tract infections in J N M C Hospital Aligarh, India. Ann Clin Microbiol Antimicrob 6: 4.
- Kothari A, Sagar V (2008) Antibiotic resistance in pathogens causing community-acquired urinary tract infections in India: a multicenter study. J Infect Dev Ctries 2: 354-358.
- Claudia V, Francesca L, Maria PB, Gianfranco D, Pietro EV, et al. (2014) Antibiotic Resistance Related to Biofilm Formation in Klebsiella pneumoniae 3: 743-758.
- Kashef N, Djavid GE, Shahbazi S (2010) Antimicrobial susceptibility patterns of community- acquired uropathogens in Tehran, Iran. J Infect Dev Ctries. 4: 202-206.
- Karlowsky JA, Jones ME, Thornsberry C, Critchley I, Kelly LJ, et al. (2001) Prevalence of antimicrobial resistance among urinary tract pathogens isolated from female outpatients across the US in 1999. Int J Antimicrob Agents 18: 121-127.
- Rajalakshmi V, Amsaveni V (2011) Antibiotic susceptibility of bacterial pathogens isolated from diabetic patients. International Journal of Microbiological Research 2: 273-275.
- Sharifian M, Karimi A, Tabatabaei SR, Anvaripour N (2006) Microbial sensitivity pattern in urinary tract infections in children: a single center experience of 1,177 urine cultures. Jpn J Infect Dis 59: 380-382.
- Haghi-Ashteiani M, Sadeghifard N, Abedini M, Soroush S, Taheri-Kalani M, et al. (2007) Etiology and antibacterial resistance of bacterial urinary tract infections in children’s medical center, Tehran, Iran. Acta medica Iranica 45: 153-157.
- Rashed marandi FRM, Saremi M (2008) A survey on urinary pathogens and their antimicrobial susceptibility among patients with significant bacteriuria. Iran J Pathol 3: 191-196.
- Dimitrov TS, Udo EE, Awni F, Emara M, Passadilla R, et al. (2004) Etiology and antibiotic susceptibility patterns of community-acquired urinary tract Infections in a Kuwait Hospital. Med Princ Pract 13: 334-339.
- Omigie O, Okoror L, Umolu P, Ikuuh G (2009) Increasing resistance to quinolones: a four-year prospective study of urinary tract infection pathogens Int. J Gen Med 2: 171-175.
- Orrett FA, Shurland SM (1998) The changing patterns of antimicrobial susceptibility of urinary pathogens in Trinidad. Singapore Med J 39: 256-259.
- Gruneberg RN (1980) Antibiotic sensitivities of urinary pathogens, 1971–1978. J Clin Pathol 33: 853-856.
- Daza R, Gutiérrez J, Piédrola G (2001) Antibiotic susceptibility of bacterial strains isolated from patients with community-acquired urinary tract infections. Int J Antimicrob Agents 18: 211-215.
- Abubakar EM (2009) Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infections at the Specialist Hospital, Yola, Adamawa State, Nigeria. J Clin Clin Med Res 1: 001-008.
- Ehinmidu JO, Bolaji RO, Adegboye EEA (2003) Isolation and antibiotic susceptibility profile of Neisseria gonorrhoeae isolated from urine samples in Zaria, northern Nigeria. J Phytomed and Ther 8: 20-24.
- Aboderin OA, Abdu L-R, Odetoyin BW, Lamikanra A (2009) Antimicrobial resistance in Escherichia coli strains from urinary tract infections. J Natl Med Assoc 101: 1268-1273.
- Haghi-Ashteiani M, Sadeghifard N, Abedini M, Soroush S, Taheri-Kalani M, et al. (2007) Etiology and antibacterial resistance of bacterial urinary tract infections in children’s medical center, Tehran, Iran. Acta Medica Iranica 45: 153-157.
- Gales AC, Jones RN, Gordon KA, Sader HS, Wilke WW, et al. (2000) Activity and spectrum of 22 antimicrobial agents tested against urinary tract infection pathogens in hospitalized patients in Latin America: report from the second year of the SENTRY AntimicrobialSurveillance Program (1998) J Antimicrob Chemother 45: 295-303.
- Abubakar EM (2009) Antimicrobial susceptibility pattern of pathogenic bacteria causing urinary tract infections at the Specialist Hospital, Yola, Adamawa State, Nigeria. J Clin Med Res 1: 001-008.
- Al Sweih N, Jamal W, Rotimi VO (2005) Spectrum and antibiotic resistance of uropathogens isolated from hospital and community patients with urinary tract infections in two large hospitals in Kuwait. Med Princ Pract 14: 401-407.
- Uwaezuoke JC, Ogbulie N (2006) Antibiotic sensitivity pattern of urinary tract pathogens in Port-Harcourt, Nigeria. J. Appl. Sci. Environ. Mgt 10: 103-107.
- Selvakumar BN, Jasmine R (2007) Antibiotic susceptibility of ESBL-producing urinary isolates at a Tertiary Care Hospital in Tiruchirappalli South India. J Biol Med Sci 7: 443-446.
- Bahadin J, Teo SSH, Mathew S (2011) Aetiology of community-acquired urinary tract infection and antimicrobial susceptibility patterns of uropathogens isolated. Singapore Med J 52 : 415-420.
- Bano K, Khan J, Begum RH, Begum H, Munir S, et al. (2012) Patterns of antibiotic sensitivity of bacterial pathogens among urinary tract infections (UTI) patients in a Pakistani population. Afr J Microbiol Res 6: 414-420.
- Rajalakshmi V, Amsaveni V (2012) Antibiotic susceptibility of bacterial pathogens isolated from diabetic patients. J Microbiol Res 3: 30-32.
- Orrett FA (2001) Urinary tract infections in general practice in a rural community in South Trinidad Saudi Med J 22: 537-540.
- García-Morúa A, Hernández-Torres A, Salazar-de-Hoyos JL, Jaime-Dávila R, Gómez-Guerra LS, et al. (2009) Community acquired urinary tract infection etiology and antibiotic resistance in a Mexican population group. Rev Mex Urol 69: 45-48.
- Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, et al. (2009) Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48: 1-12.
- Henry Oladeinde B, Omoregie R, Olley M, Anunibe JA (2011) Urinary tract infection in a rural community of Nigeria. N Am J Med Sci 3: 75-77.
- Sood S, Gupta R (2012) Antibiotic resistance pattern of community acquired uropathogens at a tertiary care hospital in Jaipur, Rajasthan. Indian J Community Med 37: 39-44.
- Nys S, van Merode T, Bartelds AIM, Stobberingh EE (2006) Urinary tract infections in general practice patients: diagnostic tests versus bacteriological culture. J Antimicrob Chemother 57: 955-958.
- Nys S, Van Merode T, Bartelds AIM, Stobberingh EE (2006) Antibiotic treatment and resistance of unselected uropathogens in the elderly. Int J Antimicrob Agents 27: 236-241.
- Nys S. Microbiology. Maastricht, The Netherlands: University Maastricht; 2005. Antibiotic resistance and commensal flora; pp. 142.
- Lipsky BA (1989) Urinary tract infections in men. Epidemiology, pathophysiology, diagnosis and treatment. Ann Intern Med 110: 138-150.
- Lipsky BA (1999) Prostatitis and urinary tract infection in men: what’s new; what’s true? Am J Med 106: 327-334.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences