ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Porous Perovskite Materials in Catalysis
Hamidreza Arandiyan* and Yuan Wang
School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
- Corresponding Author:
- Hamidreza Arandiyan
Particles and Catalysis Research Group,
School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia.
Tel: +61-2-9385-7994
E-mail: h.arandiyan@unsw.edu.au
Received Date: January 02, 2017; Accepted Date: January 03, 2017; Published Date: January 09, 2017
Citation: Arandiyan H, Wang Y. Porous Perovskite Materials in Catalysis. Synth Catal. 2017, 2:1.
Editorial
Perovskite-type oxides with the general formula ABO3 containing both rare earth elements and 3d transition metals have received intensive attention in recent years due to their wide range of applications in adsorption, separation, catalysis, and sensors. In terms of the heterogeneous catalysis, the reaction always involves diffusion and adsorption of reactants on the surface of catalyst, surface reaction of adsorbed species and desorption of products. Clearly, the reactions happened on the surface of catalyst; therefore making surface area as a critical factor to determine the availability of catalytic sites. Consequently, heterogeneous catalysis can be improved through the incorporation of improved specific surface area via engineered porosity in a solid catalyst. Hence, new strategies and technologies are continuously being developed for the synthesis and structure-tailoring of high surface area of mesoporous materials.
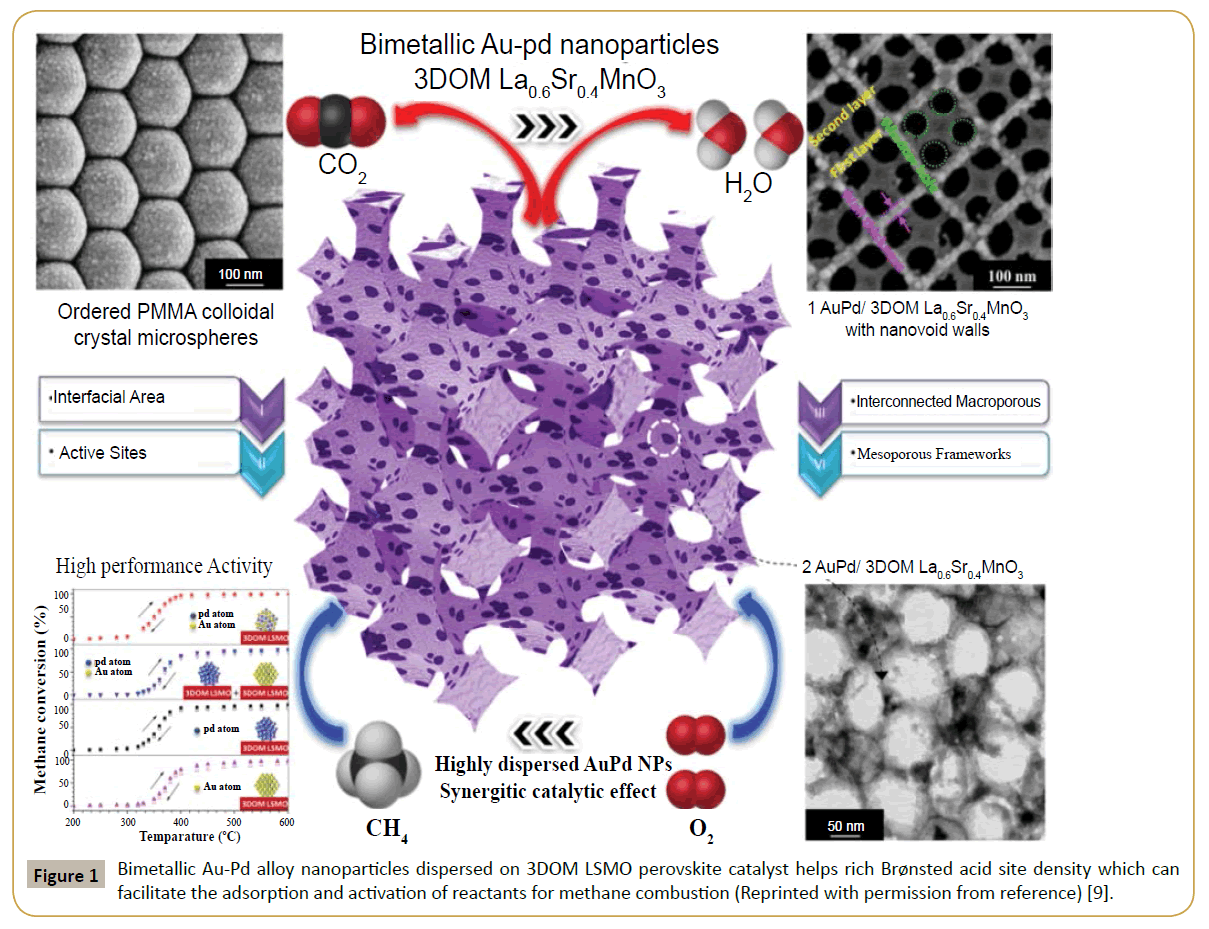
The conventional preparation of ABO3 involving a hightemperature solid-state reaction of its metal precursors usually results in a low specific surface area [1-3]. Therefore, a novel strategy is highly demanded to fabricate porous ABO3 materials with high surface areas. Recently, this problem has been solved using the close-packed Colloidal Crystal Template (CCT) method for the preparation of three-dimensionally ordered macroporous (3DOM) perovskite. The CCT which are usually formed by ordering monodispersed microspheres poly(methyl methacrylate) (PMMA), polystyrene or silica are always used to replicate the face-centered close-packed array. With PMMA microspheres as template, Arandiyan et al. [4] synthesized 3DOM-structured La1–xCexCoO3 (0>x>0.9) with a mesopore diameter of 10 nm and surface areas of 16.5–35.4 m2/g, where the 3D ordered macro/ mesoporous La0.7Ce0.3CoO3 sample exhibited high catalytic activity (T90%=555°C) for CH4 oxidation. Earlier, Dai’s research group adopted the PMMA-templating and Poly(Vinyl Alcohol) (PVA) or Polyvinylpyrrolidone (PVP)-assisted reduction approaches to successfully generate a range of 3DOM material-supported Au NPs, for example, Au/3DOM Mn2O3 (surface area=34–38 m2/g), [5] Au/3DOM La0.6Sr0.4MnO3 (surface area=31–32 m2/g), [6] Au/3DOM LaCoO3 (surface area=24–29 m2/g) [7], and Au/3DOM Co3O4 (surface area=33–36 m2/g) [8]. Very recently, Wang et al. [9] investigated Au–Pd alloy nanoparticles (1-3 wt%) dispersed on nanohybrid 3DOM La0.6Sr0.4MnO3 (LSMO) perovskite catalysts as novel distinctive class of materials and found to exhibit much higher activity than previously reported monometallic counterparts towards methane combustion. They discovered new functionalised hybrid catalysts within the temperature required for emission control. The study not only advanced knowledge in material chemistry, but also leaded to breakthroughs in the area of engineering hybrid to reduce emission and generate environmental benefits.
Bimetallic AuPd/3DOM LSMO catalysts with high surface areas for methane oxidation were successfully fabricated (Figure 1). The unique AuPd/3DOM LSMO catalysts exhibit excellent thermal stability and resistance to deactivation by water vapor during methane combustion. These bimetallic catalysts, possessing a unique 3DOM structure and Au-Pd alloy arrangement, giving excellent catalytic activity which was found to be a combined effect of both the support and Au-Pd NPs including: (i) The high surface area, (ii) Richness in adsorbed oxygen, (iii) Oxidized noble metal species on the surface, (iv) Modified electronic structure of the Au–Pd alloy, (v) Low-temperature reducibility, and (vi) Strong interaction between the Au–Pd alloy and the 3DOM support. The distinctive 3DOM structure, stable NP size and electron interaction within the bimetallic alloy allow for optimized thermal and hydrothermal stability displayed by the AuPd/3DOM LSMO catalysts. The 3DOM technique can be extended to the controlled preparation and stabilization of other nanomaterials with broad applications.
Figure 1: Bimetallic Au-Pd alloy nanoparticles dispersed on 3DOM LSMO perovskite catalyst helps rich Brønsted acid site density which can facilitate the adsorption and activation of reactants for methane combustion (Reprinted with permission from reference) [9].
References
- Arandiyan H, Peng Y, Liu C, Chang H, Li J (2014) Effects of noble metals doped on mesoporous LaAlNi mixed oxide catalyst and identification of carbon deposit for reforming CH4 with CO2. J Chem TechnolBiotechnol 89: 372-381.
- Arandiyan H, Parvari M (2008) Preparation of La-Mo-V mixed-oxide systems and their application in the direct synthesis of acetic acid. J Natural Gas Chem 17: 213-224.
- Arandiyan H, Parvari M (2009) Studies on mixed metal oxides solid solutions as heterogeneous catalysts. Braz J Chem Eng 26: 63-74.
- Arandiyan H, Scott J, Wang Y, Dai H, Sun H (2016) Meso-Molding Three-Dimensional MacroporousPerovskites: A New Approach to Generate High-Performance Nanohybrid Catalysts. ACS Appl Mater Interfaces 8: 2457-2463.
- Xie S, Dai H, Deng J, Yang H, Han W, et al. (2014) Preparation and high catalytic performance of Au/3DOM Mn2O3 for the oxidation of carbon monoxide and toluene. J Hazard Mater 279: 392-401.
- Liu Y, Dai H, Deng J, Li X, Wang Y, et al. (2013) Au/3DOM La0.6Sr0.4MnO3: Highly active nanocatalysts for the oxidation of carbon monoxide and toluene. J Catal 305: 146-153.
- Li X, Dai H, Deng J, Liu Y, Xie S, et al. (2013) Au/3DOM LaCoO3: High-performance catalysts for the oxidation of carbon monoxide and toluene. Chem Eng J 228: 965-975.
- Xie S, Deng J, Zang S, Yang H, Guo H, et al. (2015) Au–Pd/3DOM Co3O4: Highly active and stable nanocatalysts for toluene oxidation. J Catal 322: 38-48.
- Wang Y, Arandiyan H, Scott J, Akia M, Dai H, et al. (2016) High Performance Au–Pd Supported on 3D Hybrid Strontium-Substituted Lanthanum Manganite Perovskite Catalyst for Methane Combustion. ACS Catal 6: 6935-6947.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences