ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Multi-wall Carbon Nanotubes Decorated with Barium Oxide Nanoparticles
Arvind K. Bhakta1, Ronald J. Mascarenhas2, Praveen Martis3, Joseph Delhalle1 and Zineb Mekhalif1*
1Laboratory of Chemistry and Electrochemistry of Surfaces, University of Namur, 61 Rue de Bruxelles, B-5000 Namur, Belgium
2Department of Chemistry, Electrochemistry Research Group, St. Joseph's College, Lalbagh Road, Bangalore 560 027, Karnataka, India
3Loyola Centre for Research and Innovation, St. Aloysius College, Light House Hill Road, Mangalore 575003, Karnataka, India
- *Corresponding Author:
- Zineb Mekhalif
Laboratory of Chemistry and Electrochemistry of Surfaces
University of Namur
61 Rue de Bruxelles, B-5000 Namur, Belgium
Tel: +32(0)81725230
E-mail: zineb.mekhalif@unamur.be
Received Date: January 01, 2018; Accepted Date: February 02, 2018; Published Date: February 07, 2018
Citation: Bhakta AK, Mascarenhas RJ, Martis P, Delhalle J, Mekhalif Z (2018) Multi-wall Carbon Nanotubes Decorated with Barium Oxide Nanoparticles. Synth Catal. 3:1. doi: 10.4172/2574-0431.100019
Abstract
A simple, reproducible and efficient technique to decorate multi-wall carbon nanotubes (MWCNTs) with barium oxide nanoparticles (BaO NPs) using infrared (IR) irradiation is developed. NaOH treatment leads to the purification of MWCNTs (p-MWCNTs). Functionalizing p-MWCNTs with tricarboxylic aryl diazonium salts generated in-situ and then reacting it with barium acetate in the presence of IR irradiation is the key step in efficiently impregnating p-MWCNTs-D3 with barium acetate (p-MWCNTs-D3/BA). Materials are characterized using XPS, TEM and PXRD. Homogeneous distribution of BaO NPs on MWCNTs is evidenced, with a Gaussian mean diameter of 5.1 nm. This method is also applicable to large scale preparation which opens interesting perspectives for nanotechnology applications.
Keywords
Multi-wall carbon nanotubes; Barium oxide nanoparticles; Sonication; Infrared irradiation; Nanocomposite
Introduction
Carbon nanotubes (CNTs) are among the most effective allotropes of carbon. They exhibit very interesting properties such as high chemical stability, extraordinary tensile strength, desired electrical and thermal conductivities and large surface area [1]. These properties help CNTs to find potential applications in the field of chemical and biosensors, hydrogen storage, solar and fuel cells, supercapacitors, and lithium ion batteries [2]. Crude CNTs not only contain impurities such as alumina (residual impurities from the synthesis process), but they are also difficult to solubilize in most of the solvents. These problems can be solved by purification and functionalization of the CNTs [3]. Nanoscale dimensions providing high surface area make them to be considered as efficient templates for the assembly of nanoparticles. In the current years, substantial interest has been noted for metal or metal oxide nanoparticles decorated carbon nanotube hybrid materials [4].
Alkaline earth metal oxides have been identified as materials which catalyze many types of reactions. BaO is a catalyst with strong basic sites which play very important role in isomerization and co-isomerization reactions [5]. It also catalyzes transesterification reactions [6]. Barium oxide (BaO) is an important direct band gap II–VI semiconductor material having a large band gap (4.4 eV), robust mechanical strength, high thermal stability and oxidation resistance in harsh environments [7]. It is a potential candidate for field emission devices [8] , hydrogen separation membranes [9] and gas storage materials, to cite a few. Amorphous barium oxide plays a very important role in solid oxide fuel cells [10].
CNTs absorb radiation near- infrared (IR) area, rapidly transferring electronic excitations into molecular vibration energies which produce heat. These interesting properties: photo-absorption and photo-thermal have been also used for CNTs decoration [11].
To the best of our knowledge, there is an only one work [12] reporting on the decoration of CNTs with barium oxide nanoparticles. However, this method suffers from limitations such as very large particles size and very low concentration of particles on CNTs. In the present method, we have successfully controlled the size, nature and distribution of BaO NPs on MWCNTs. The present method using IR irradiation to decorate CNTs with BaO NPs is simple. Thus, present methodology is advancing the science by substantially improving the reported work. Also, our strategy has not been reported before for Ba, hence the motivation for this work. The nanocomposite material as a result of combination of MWCNTs and BaO NPs can lead to effective integration of properties of both constituents in new hybrid material which is important for the nanotechnology applications, nanocatalysis, for example.
Materials and Methods
Chemicals
All the chemicals are of analytical grade or higher purity. The thin MWCNTs (NC 7000) (>95%) purchased from Nanocyl SA (Belgium) have an average diameter of 10 nm with lengths ranging from 0.1 – 10 μm. Barium acetate (99%) is purchased from MERCK. All aqueous solutions are prepared using ultra-pure water.
Apparatus
XPS spectra are carried out on a Thermo Scientific K-Alpha spectrometer using monochromatized Al Kα radiation (1486.6 eV). Transmission electron microscopy (TEM) studies are carried out using Tecnai 10 philips microscope, operating at 80 kV accelerating voltage and 5 μA emission current. Powder X-Ray diffraction (PXRD) analysis is performed using PAN analytical XPert PRO Bragg-Brentano diffractometer with tube current of 30 mA and an operating voltage of 45 kV with Cu Kα (λ= 1.5418 Å). Irradiation of the samples are carried out using a Petra IR 11 IR lamp
Purification of crude MWCNTs and tricarboxylic aryl diazonium functionalization of p-MWCNTs
The process of purification and tricarboxylic aryl diazonium functionalization of MWCNTs are carried out using the method reported in the literature [13]. The purified MWCNTs and tricarboxylic aryl diazonium functionalized MWCNTs are referred to as p-MWCNTs and p-MWCNTs-D3, respectively.
Impregnation of barium acetate on p-MWCNTs-D3
Mixture of an aqueous solution (100 ml) of 0.255 g of barium acetate (BA) and the functionalized CNTs (p-MWCNTs-D3) are sonicated for 5 minutes and then IR irradiated for 2 hours under constant magnetic stirring. The mixture is cooled down to room temperature, filtered and the residue is washed with water then by acetone and finally dried in air. The impregnated p-MWCNTs thus obtained are referred to hereafter as p-MWCNTs-D3/BA.
Calcination of p-MWCNTs-D3/BA
The p-MWCNTs-D3/BA are calcined in a furnace at 400°C for 2 h under a continuous flow of argon gas. The achieved material is labeled as p-MWCNTs/BaO
Results and Discussion
Carbon nanotubes are characterized by XPS, TEM and PXRD. They are compared at each step of their modifications i.e. purification, functionalization, impregnation and calcination.
Materials chemical composition by XPS
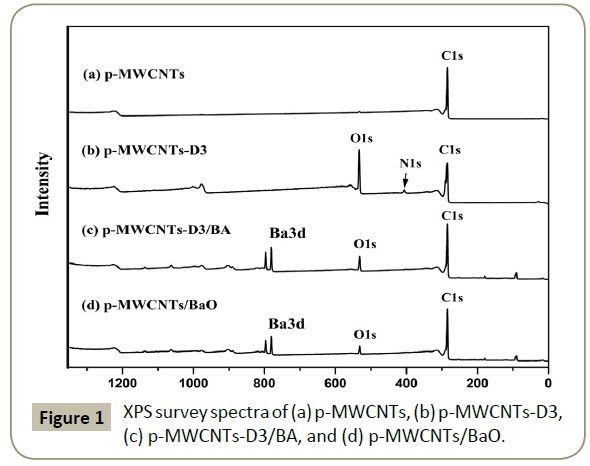
XPS was carried out to assess the chemical composition of the different samples and the results are displayed in Table 1. XPS general survey spectra of p-MWCNTs, p-MWCNTs-D3, p-MWCNTs-D3/BA, and p-MWCNTs/BaO are shown in Figure 1. The absence of alumina in p-MWCNTs (Figure 1a) is indicative of the purity of the sample and the efficiency of the purification method. The increase in the amount of O1s and the presence of a N1s peak (Figure 1C) proves that the MWCNTs are functionalized with the tricarboxylic aryl diazonium group. Furthermore, the presence of barium (Figure 1c) indicates the successful impregnation of barium acetate on the tricarboxylic functionalized MWCNTs. As expected, the Ba peak exists after calcination (Figure 1d) but there is a decrease in the amount of barium from the impregnation to the calcination steps.
| Materials | C% | O% | N% | Al% | Ba% |
|---|---|---|---|---|---|
| crude MWCNTs | 97.18 | 1.98 | - | 0.84 | - |
| p-MWCNTs | 98.27 | 1.73 | - | - | - |
| p-MWCNTs-D3 | 71.97 | 24.89 | 3.14 | - | - |
| p-MWCNTs-D3/BA | 86.08 | 10.83 | 1.15 | - | 1.94 |
| p-MWCNTs/BaO | 92.71 | 5.65 | - | - | 1.64 |
Table 1: Chemical composition of different materials obtained from XPS analysis.
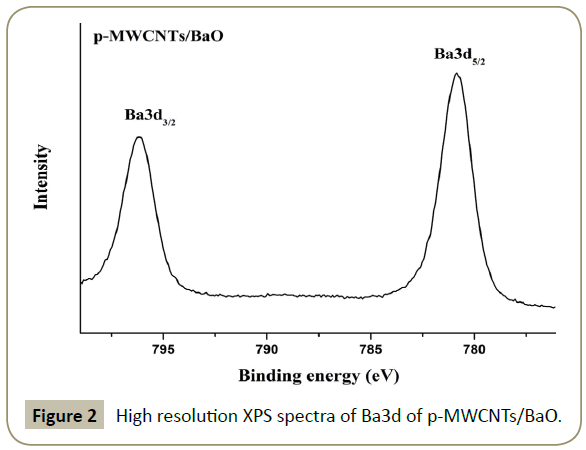
The high resolution Ba3d XPS spectrum of p-MWCNTs/BaO is displayed in Figure 2. The Ba3d5/2 and Ba3d3/2 are formed at 780.69 and 795.97 eV, indicating the presence of Ba in +2 oxidation state, which agrees well with the literature [14].
Materials morphology by TEM
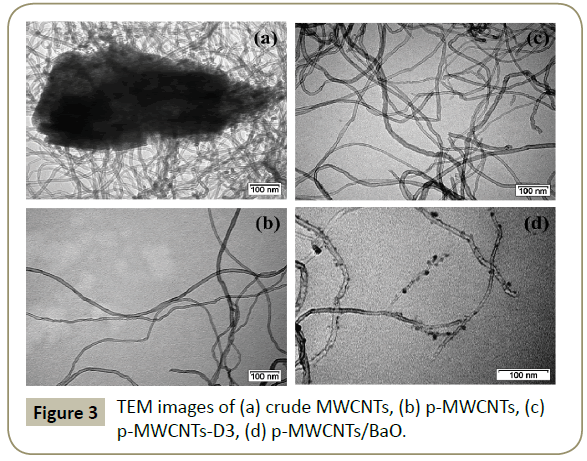
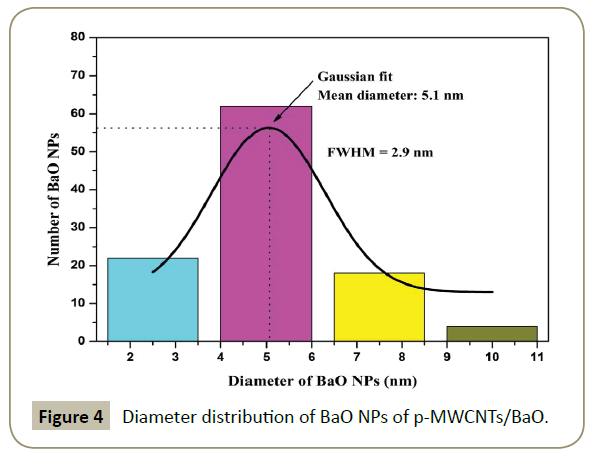
Figure 3 shows the TEM images of the prepared materials. TEM micrographs of crude MWCNTs (Figure 3a) point to a substantial amount of alumina (impurity) which is eliminated in the p-MWCNTs (Figure 3b). This evidences the effectiveness of the purification method. (Figure 3c) is a TEM image of tricarboxylic aryl diazonium functionalized MWCNTs. Nanotubes are intact even after the purification and functionalization steps. This evidences the effectiveness of the purification and functionalization methods. This is a definite advantage over acid treatments which causes severe damages to the tubes [15]. As shown in TEM images (Figure 3d), small size BaO NPs, with a Gaussian mean diameter of ∼5.1 nm (Figure 4) are uniformly distributed over CNTs surface. No BaO NPs are detached from the MWCNTs indicating that they are strongly anchored on the CNTs surface.
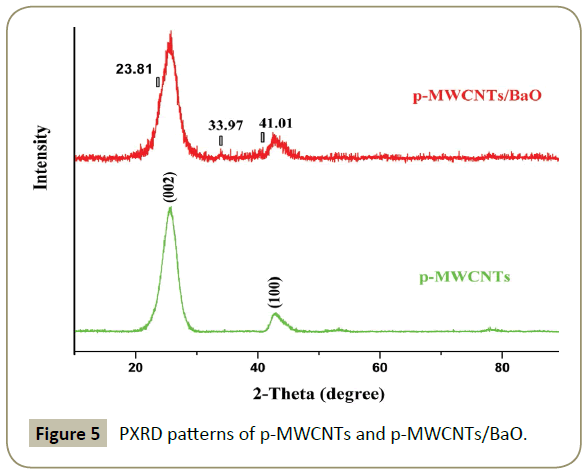
Characterization of materials by PXRD
PXRD patterns of p-MWCNTs and p-MWCNTs/BaO are displayed in (Figure 5). In all the samples, the diffraction peak at 2θ=25.66° is due to the reflection from (002) plane of graphitic carbon forming the MWCNTs structure. The diffraction patterns of the sample p-MWCNTs/BaO match with those of barium oxide nanoparticles decorated CNTs reported in the literature [12] indicating that the nanoparticles formed in our case are barium oxide nanoparticles.
This is in accordance with XPS data. The obtained peak is not sharp indicating that the NPs are amorphous in nature. Amorphous BaO NPs are very important for nanotechnology applications [10].
Conclusions
We are reporting a simple method for decorating MWCNTs with BaO nanoparticles (NPs) by making use of diazonium chemistry and infrared irradiation. These NPs exhibits Gaussian mean diameter of ∼5.1 nm. NPs (amorphous nature) are uniformly distributed over the MWCNTs surface. As BaO is very usefull in many fields, the hybrid material obtained from MWCNTs and BaO NPs could lead to the successful integration of properties of both the constituents in the new nanocomposite which will play important role in catalysis and nanotechnology.
Though there are still some limitations of the present method since the results are not as promising compared to results reported for other nanoparticles [13] but it advances the science by substantially improving the method for decorating CNTs with BaO NPs. Some modifications such as varying the nature of salt, duration of IR irradiation, solution of process etc. in the present approach can be done to further improve the results.
Acknowledgement
Arvind K. Bhakta thanks the University of Namur for a CERUNA doctoral fellowship.
Conflict of Interest
The authors declare that there is no conflict of interest.
References
- Bhakta AK, Mascarenhas RJ, D’Souza OJ, Satpati AK, Detriche S, et al. (2015) Iron nanoparticles decorated multi-wall carbon nanotubes modified carbon paste electrode as an electrochemical sensor for the simultaneous determination of uric acid in the presence of ascorbic acid, dopamine and l-tyrosine. Mater Sci Eng C Mater Biol Appl 57: 328–337.
- Zhang Y, Li K, Ji P, Chen D, Zeng J, et al. (2017) Silicon-multi-walled carbon nanotubes-carbon microspherical composite as high-performance anode for lithium-ion batteries. J Mater Sci 52: 3630–3641.
- Karousis N, Tagmatarchis N, Tasis D (2010) Current progress on the chemical modification of carbon nanotubes. Chem Rev 110: 5366–5397.
- Haniff MASM, Hafiz SM, Wahid KA, Endut Z, Syono MI, et al. (2017) Nitrogen-doped multiwalled carbon nanotubes decorated with copper(I) oxide nanoparticles with enhanced capacitive properties. J Mater Sci 52: 6280-6290.
- Hattori H, Maruyama k, Tanabe k (1976) Active sites on barium oxide for isomerization of butenes, exchange of butenes with D2, and H2-D2 equilibration. J Catal 44: 50-56.
- Martinez-Guerra E, Gude VG (2014) Transesterification of used vegetable oil catalyzed by barium oxide under simultaneous microwave and ultrasound irradiations. Energy Convers Manag 88: 633-640.
- Cui Y, Chen J, Zhao D, Zhang X, Lei W, et al. (2014) Stable field emission lamps based on well-aligned BaO nanowires. RSC Adv 4: 22246.
- Cui Y, Chen J, Zhang Y, Zhang X, Lei W, et al. (2017) Di Enhanced performance of thermal-assisted electron field emission based on barium oxide nanowire. Appl Surf Sci 396: 1108–1112.
- Siriwardane RV, Poston JA, Fisher EP, Lee TH, Dorris SE, et al. (2003) Characterization of ceramic-metal composite hydrogen separation membranes consisting of barium oxide, cerium oxide, yttrium oxide and palladium. Appl Surf Sci 217: 43–49.
- Song Y, Wang W, Ge L, Xu X, Z Zhang, et al. (2017) Rational Design of a Water-Storable Hierarchical Architecture Decorated with Amorphous Barium Oxide and Nickel Nanoparticles as a Solid Oxide Fuel Cell Anode with Excellent Sulfur Tolerance. Adv Sci 4: 17003371-378.
- Martis P, Venugopal BR, Seffer JF, Delhalle J, Mekhalif Z (2011) Infrared irradiation controlled decoration of multiwalled carbon nanotubes with copper / copper oxide nanocrystals. Acta Mater 59: 5040-5047.
- Hasnahena ST, Satpati B, Roy M (2016) Enhanced sensing of NH3 gas by decorated multiwalled carbon nanotube. AIP Conf Proc 1731: 0500951–953.
- Bhakta AK, Detriche S, Martis P, Mascarenhas RJ, Delhalle J, et al. (2017) Decoration of tricarboxylic and monocarboxylic aryl diazonium functionalized multi-wall carbon nanotubes with iron nanoparticles. J Mater Sci 52: 9648–9660.
- Ji F, Shi F, Wang S, Teng S, Jian S (2012) Effects of annealing temperatures on crystalline quality of ceramic thin films by RF-magnetron sputtering using Zn-enriched (Ba0.3Sr0.7)(Zn1/3Nb2/3)O3 as target. J Mater Sci Mater Electron 23: 164-168.
- Chen J, Hamon MA, Hu H, Chen Y, Rao AM, et al. (1998) Solution Properties of Single-Walled Carbon Nanotubes. Science 282: 95-98.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences