ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Montmorillonite K10 Catalysed Condensation of 1-(3-Chlorophenyl)-4-(3-chloropropyl) piperazine and 1,2,4-Triazolo [4,3-a]pyridine-3-(2H)-one: A Proficient and Green Synthesis of Trazodone Hydrochloride and Its Analogues
1Department of Chemistry, Manoharbhai Patel College of Arts, Commerce and Science, Deori, Maharashtra, India
2Organo-Analytical Division, Department of Chemistry, Government Institute of Science, Nagpur, Maharashtra, India
- Corresponding Author:
- Trimurti Laxmikant Lambat
Department of Chemistry, Manoharbhai Patel College of Arts, Commerce and Science, Deori, Gondia-441901, Maharashtra, India.
Tel: +918007011077
Fax: +917199225180
E-mail: lambatmbpc@gmail.com
Received Date: November 16, 2016; Accepted Date: November 30, 2016; Published Date: December 05, 2016
Citation: Lambat TL, Deo SS. Montmorillonite K10 Catalysed Condensation of 1-(3-Chlorophenyl)-4-(3- chloropropyl)piperazine and 1,2,4-Triazolo [4,3-a]pyridine-3-(2H)-one: A Proficient and Green Synthesis of Trazodone Hydrochloride and Its Analogues. Synth Catal. 2016, 1:1.
Abstract
Montmorillonite K10 clay has an extremely significant heterogeneous catalyst for condensation reaction with high yield of the product as compared to other reported reagents.
Background: Novel green, synthetic methodology has been adopted for the synthesis of 2-[3-{4-(3-chlorophenyl)-1-piperazinyl}propyl]-1,2,4-triazolo[4,3-a] pyridine-3-(2H)-one hydrochloride (5) analogues using Montmorillonite K10 (MK10) as an effective-efficient heterogeneous catalyst. The present synthesis offers various significant features with numerous advantages such as outstanding yields, operational simplicity, recyclability and reusability of the catalyst for this synthetic reaction.
Methods and findings: Condensation reaction is the only exceptional tool for the synthesis of numerous biologically significant organic compounds. In the current investigation, condensation of 1-(3-chlorophenyl)-4-(3-chloropropyl) piperazine (3) and 1,2,4-triazolo[4,3-a]pyridine-3-(2H)-one (4) in the presence of MK10 under thermal condition has been carried out with afford the corresponding products i.e., (5) analogues with good yields (88-95%) over conventional reagents. MK10 is such a heterogeneous catalyst which can be completely recovered and reusable for a three times without potential loss in its catalytic activity.
Keywords
Condensation reaction; Montmorillonite K10; Recyclability; Heterogeneous catalyst
Introduction
Multi component condensation reactions [1] that are performed in heterogeneous catalyst have gained improved interest in synthetic chemistry over the past decade not only for the advantages rendered by avoiding extensive decomposition of reactants, strong reaction conditions and solvents, but also for the development of environmentally benign methodology [2].
Furthermore, when a heterogeneous catalyst [3] is used, the insoluble catalyst can be separated by simple filtration and the catalyst can be reused. Therefore, the improvement of a heterogeneous catalyst in solvent appears enormously desirable.
Commercially available Montmorillonite K10 (MK10) [4] is one such catalyst that can fulfill these requirements. MK10 are environmentally friendly and economically reasonable solid acid catalysts that offers several advantages, such as ease of handling, non-corrosiveness, low cost and regeneration. Green chemistry, believes in replacement of toxic organic solvents [5], which are responsible for excessive waste of chemicals and environmental problems. In response catalysts such as MK10 are presently under dynamic research. These not only avoid the use of toxic acids, high temperature carrying reactions but also prompt important simplifications to the reaction procedures. The main contributing factors are the high atom economy, extensive application in combinatorial chemistry and diversity oriented synthesis [6-10].
The synthetic product (5), known as “Trazodone hydrochloride” [11] is a well-known drug with psychoactive properties synthesized from piperazine and triazolopyridine classes of compounds having antidepressant, anxiolytic and hypnotic properties [12]. The pharmacological potential of Trazodone hydrochloride is due to inhibition of serotonin uptake and also a lower affinity for the serotonin transporter than other drugs which belongs to selective serotonin reuptake inhibitor class [13].
Recently, the Synthesis of Novel Benzofluorenone Derivatives and their HIV-Reverse Transcriptase Inhibitory Activity [14], one-pot synthesis of new N,N’-alkylidene bisamide derivatives under solvent free condition by montmorillonite K10 [15] and the synthesis of Trazodone hydrochloride by sulphamic acid are reported [16].
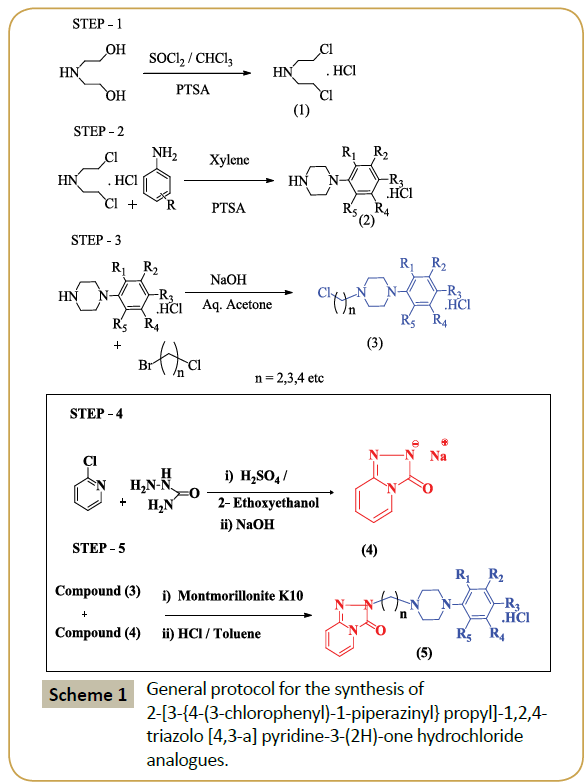
Thus, in the present study the green synthetic procedure for the synthesis of (5) analogues in the presence of MK10 as an efficient catalyst has been established (Scheme 1). The literature survey reveals that there is reported no prior synthesis of these new compounds using green synthetic methodology.
Methods
Materials and reagents
Required chemicals and reagents were procured from Aldrich (Sigma–Aldrich, St. Louis, MO, USA), Lancaster (Alfa Aesar, Johnson Matthey Company, Ward Hill, MA, USA) and used without further purification. Reaction monitoring was done with Thin Layer Chromatography (TLC), performed on silica glass plates 60 GF-254, and visualization was achieved by UV light. Column chromatography was performed by Merck 60–120 mesh silica gel. 1H spectra were recorded by Bruker UXNMR/XWINNMR (300 MHz) instruments. Chemical shifts (δ) were reported in ppm downfield from internal TMS standard. ESI spectra were recorded in Micro mass; Quattro LC uses ESI+ software with capillary voltage 3.98 kV and ESI mode positive ion trap detector. Melting points were determined with an Electro thermal melting point apparatus, and were approximate.
Novel procedure for synthesis of Intermediate analogues
Intermediate analogues were synthesized based on practically a similar procedure as reported by Lambat and Deo [16]:
Step 1: General procedure for the synthesis of bis-(2- chloroethylamine) hydrochloride (1) [16].
Step 2: General procedure for the synthesis of 1-(3-chlorophenyl)- piperazine hydrochloride and its derivatives (2) [16].
Step 3: General procedure for the synthesis of 1-(3-chlorophenyl)- 4-(3-chloropropyl) piperazine and its derivatives (3) [16].
Step 4: General procedure for the synthesis of sodium salt of 1, 2, 4-triazolo [4, 3-a] pyridine-3-(2H)-one (4) [16].
Step 5: Novel procedure for the synthesis of Trazodone hydrochloride derivative.
In a typical reaction procedure, product (3) (3.6 mmol), (4) (1.15 mol) and Montmorillonite K10 (0.500 g) in acetonitrile (3.00 mL). The resulting mixture was refluxed at 90°C for 5 h. After completion of the reaction as monitored by TLC, the reaction mixture was cooled to room temperature and filtered. The acetonitrile was removed by distillation and toluene (3 mL) was added to the reaction mixture and stirred for 10 min and filtered. Then, the catalyst was isolated by simple filtration, which was recovered later and the remaining supernatant was treated with 20% NaOH followed by 2% brine solution at 50°C. The toluene solution containing desired product which is base, in which HCl (15%) was added and pH adjusted between 2-2.5 when salt starts precipitating. The precipitated product was filtered; recrystallization of the crude product from methanol to furnished the pure product. All the synthesized products (5A-5G) are known compounds, which were characterized based on their melting points and spectral (FT-IR, 1H and 13C NMR) data and compared with the reported corresponding data.
Spectral data of synthesized product
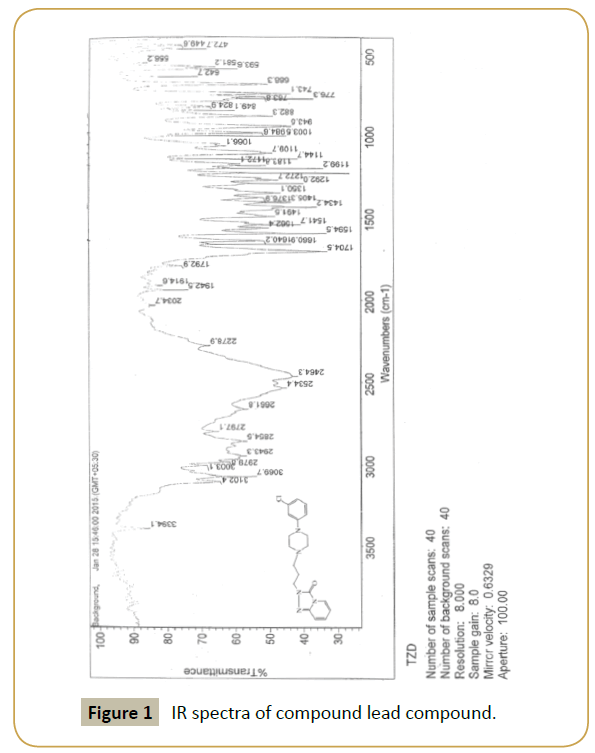
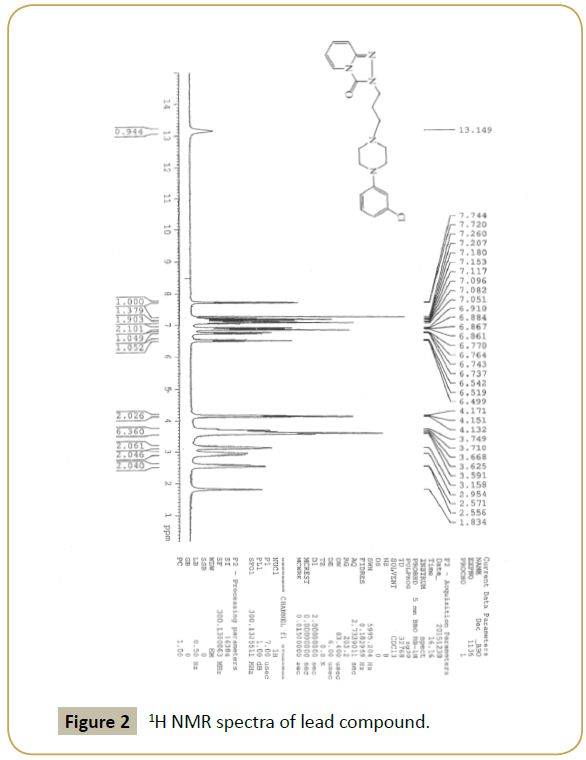
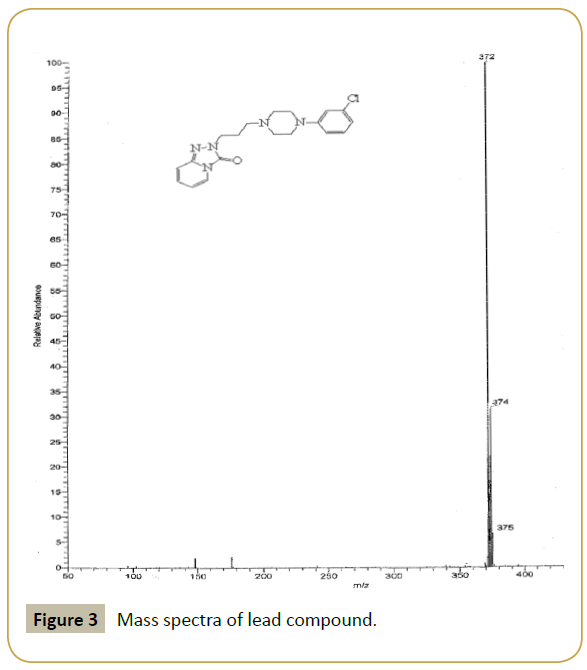
Lead compound: IR (KBr) (νmax): 3000, 2954, 1704, 1650, 1600, 1350.80, 750; 1H NMR(300 MHz, DMSO-d6) δ: 2.16-2.12 ppm (t, 2H, N-CH2-CH2-CH2-N), 2.64-2.60 (t, 2H, N-CH2), 2.73 (s, 4H, -CH2- N-CH2), 3.09 (s, 4H, CH2-N-CH2), 4.12-4.07 (t, 2H,-CH2-N), 6.51- 6.46 (m,1H,-ArH), 7.02-6.93 (m, 2H,-ArH), 7.09-7.08 (d, 2H,-ArH), 7.26-7.17 (m, 1H,-ArH), 7.34-7.31 (d, 1H,-ArH) 7.76-7.74 (d, 1H,- ArH); m/z (ESI): 372 (M+).
5A: IR (KBr) (νmax): 3000, 2850, 1700, 1650, 1600, 1350, 750; 1H NMR(300 MHz, DMSO-d6) δ: 2.50-2.49 (m, 2H, -CH2), 3.21-3.15 (m, 8H, CH2-piperazine), 3.60 (t, 2H -CH2), 4.01(t, 2H, -CH2), 6.65- 6.63 (m,1H, -ArH), 7.25-7.21(m, 3H, -ArH), 7.36-7.34 (t, 2H, -ArH), 7.88-7.86 (d,1H, -Ar H); m/z (ESI): 406 (M+).
5B: IR (KBr) (νmax): 3050, 3100, 2862, 2947, 1704.96, 1643.24, 1635.23, 1350.08, 750; 1H NMR(300 MHz, DMSO-d6) δ: 2.26-2.19 (m, 2H, CH2), 3.23-3.01(m, 6H, CH2-piperazine), 3.40-3.50 (d, 2H, CH2-piperazine), 3.90-3.86 (d, 2H, CH2), 4.01-3.97 (t, 2H, CH2), 6.67-6.60 (m, 1H, Ar H), 7.01-6.97 (m, 1H, -Ar H), 7.21-7.20 (m, 3H, -Ar H), 7.40 (d,1H, -Ar H), 7.88-7.859 (d,1H, -Ar H); m/z (ESI): 406.3 (M+).
5C: IR (KBr) (νmax): 3000, 2870, 1710, 1635, 1610, 1350, 575; 1H NMR(300 MHz, DMSO-d6) δ: 2.28-2.26 (t, 2H, CH2), 3.09 (t, 2H, CH2-piperazine), 3.23-3.20 (d, 4H, CH2-piperazine), 3.53-3.51 (t,2H,CH2-Piperazine), 3.87-3,84 (d, 2H, CH2), 4.02-3.98 (t, 2H, CH2), 6.64-6.63 (t, 1H, -ArH), 6.86-6.84 (m,1H, -Ar H), 6.96-6.95 (d,1H, -Ar H), 7.04-7.03 (t,1H, -Ar H), 7.26-7.22 (m, 3H, -Ar H), 7.87-7.85 (d,1H, -ArH); m/z (ESI): 415 (M+).
5D: IR (KBr) (νmax): 3050, 2850, 2950, 1720, 1650, 1500, 1325, 1164.92; 1H NMR(300 MHz, DMSO-d6) δ: 2.31-2.24 (m, 2H, CH2), 3.20-3.12 (m, 6H, CH2-piperazine), 3.56-3.53 (d, 2H,CH2- piperazine), 3.72-3.69 (d, 2H, CH2), 4.03-3.99 (t, 2H, CH2), 6.66- 6.61(m, 1H, -Ar H), 7.13-7.00 (m, 4H, -Ar H), 7.25-7.24 (d, 2H, -Ar H), 7.88-7.86 (d,1H, -Ar H); m/z (ESI): 356 (M+).
5E: IR (KBr) (νmax): 3010, 2875, 1715, 1650, 1550, 1350.45, 775; 1H NMR(300 MHz, DMSO-d6) δ: 1.94-1.88 (m, 2H, CH2), 2.38-2.28 (m, 2H, CH2), 2.44-2.40 (s, 4H, CH2-piperazine), 3.07-3.04 (t, 4H, CH2-piperazine), 3.97-3.93 (t, 2H, CH2), 6.61-6.65 (m,1H, -Ar H), 6.90-6.97 (m, 2H, -Ar H), 7.12-7.09 (s, 1H, -Ar H), 7.25-7.15 (m,1H, -Ar H), 7.84-7.82 (d,1H, -Ar H); m/z (ESI): 441 (M+).
5F: IR (KBr) (νmax): 3000, 2947.03, 1710, 1650, 1500, 1350; 1H NMR(300 MHz, DMSO-d6) δ: 1.92-1.87 (t, 2H, CH2), 2.09-2.03 (d, 6H, -Ar-CH3), 2.50-2.36 (m, 6H, CH2 piperazine), 2.66 (s, 4H, CH2- piperazine), 3.98-3.94 (t, 2H, CH2), 6.62-6.58 (t, 1H, -Ar H), 6.82- 6.80 (d,1H, -Ar H), 6.93-6.90 (d, 2H, -Ar H), 7.25-7.10 (m, 2H,-Ar H), 7.86-7.84 (d, 1H, -Ar H); m/z (ESI): 366 (M+)
5G: IR (KBr) (νmax): 3050, 2850, 1700, 1650, 1600, 1347; 1H NMR(300 MHz, DMSO-d6) δ: 1.16-1.13 (t, 3H, CH3), 1.93-1.89 (m, 2H, CH2), 2.29 (t, 2H, CH2), 2.99-2.77 (m, 6H, CH2, CH2-piperazine), 3.17 (t, 2H, CH2), 4.12-3.93 (m, 4H, CH2- piperazine), 5.20 (m, 1H, -ArH), 5.77 (m, 1H, -ArH), 6.59-6.57 (m, 2H, ArH), 6.8 (d,1H, -ArH), 7.07 (d,1H, -ArH), 7.27-7.17 (d, 2H, -ArH); m/z (ESI): 366.53 (M+).
5H: IR (KBr) (νmax): 3132, 3050, 2862, 1710, 1650, 1500, 1430, 1350, 750; 1H NMR(300 MHz, DMSO-d6) δ: 1.16-1.13 (t, 3H, CH3), 1.93-1.89 (m, 2H, CH2), 4.02-3.97 (t, 2H, CH2), 3.19-3.02 (m, 6H, CH2-piperazine), 3.80-3.76 (t, 2H, CH2), 3.54-3.50 (d, 2H, CH2- piperazine), 6.65-6.60 (m, 1H, -ArH), 7.01-6.95 (m, 1H, -ArH), 7.18-7.17 (m, 1H, ArH), 7.25-7.19(t,2H, -ArH), 7.31-7.25(m,1H, -ArH), 7.88-7.85 (d, 2H, -ArH),11.08(broad s, 1H, -NH); m/z (ESI): 358(M+).
5I: IR (KBr) (νmax): 3105, 3000, 2850, 1700, 1650, 1600, 1431, 1350, 750; 1H NMR(400 MHz, DMSO-d6) δ: 1.40-1.20 (m, 2H, CH2), 1.60-1.41 (m, 2H, CH2), 2.30-2.15 (t, 2H, CH2), 3.02-2.74 (m, 4H, CH2-piperazine), 3.26-3.08 (t, 2H, CH2), 4.17-4.05 (m, 4H, CH2- piperazine), 6.26-6.12 (d, 1H, -ArH), 7.05-6.74 (t, 1H, -ArH), 7.11-7.05 (d, 1H, ArH), 7.26-7.11 (m, 1H, -ArH), 8.02-7.74 (m, 2H, -ArH), 8.25-8.12 (d, 1H, -ArH), 8.52-8.30(t, 1H, -ArH); m/z (ESI): 386(M+).
5J: IR (KBr) (νmax): 3152, 3050, 2850, 2925, 1700, 1650, 1535, 1416, 1325; 1H NMR(400 MHz, DMSO-d6) δ: 2.65-2.63 (t, 2H, CH2), 3.24-3.22 (m, 4H, CH2-piperazine), 3.41-3.38 (t, 2H, CH2), 3.77-3.68 (m, 4H, CH2-piperazine), 4.21-4.18 (t, 2H, CH2), 6.55 (t, 1H, -ArH), 7.21-7.11 (m, 6H, -ArH), 7.42 (s, 1H, ArH), 7.49-7.47 (m,1H, -ArH), 7.63-7.62 (d,1H, -ArH), 7.78-7.76 (d, 1H, -ArH); m/z (ESI): 388 (M+).
Results and Discussion
Firstly, bis-(2-chloroethylamine) hydrochloride has been synthesized by chlorination of diethanolamine with thionyl chloride in CHCl3, which was condensed further with many a substituted anilines to develop various analogues of 1-(3-chlorophenyl)-piperazine hydrochloride intermediate. Alkylation of these synthesized intermediates using 1-bromo-3- chloropropane in alkaline aqueous acetone (50%) gave different analogs of 1-(3-chlorophenyl)-4-(3-chloropropyl) piperazine intermediate.
In product (4) is synthesized by condensation reaction followed by subsequent cyclization of 2-chloropyridine, semicarbazide in 2-ethoxyethanol and sulfuric acid. Sulfuric acid is self-catalyst so no other catalyst is needed for this reaction, and hence the reaction proceeds without any hurdle. In this methodology, one pot synthesis of (4) was achieved in a short span of time and with excellent yields (95%).
Our laboratory has developed a novel Green chemistry route for step (5). To optimize the quantity of MK10 catalyst, (3) and (4) with MK10 in acetonitrile was selected as model reaction. Using different quantities of catalyst at 90°C for 5 h, the use of 0.500 g of catalyst resulted in the highest yield, 94% (Table 1 and Figures 1-3).
| Entry | Amount of Catalyst (g) | Timeb,c (min) |
Yield (%)a |
|---|---|---|---|
| 1 | 0.350 | 90 | 68 |
| 2 | 0.400 | 90 | 73 |
| 3 | 0.450 | 90 | 84 |
| 4 | 0.500 | 90 | 94 |
| 5 | 0.550 | 90 | 90 |
| 6 | 0.600 | 90 | 82 |
| 7 | 0.800 | 90 | 69 |
Table 1 Optimization of Montmorillonite K10 catalyst for synthesis of model product (lead compound). aIsolated yield; bReaction carried out at 90°C; cModel reaction- 1-(3-chlorophenyl)-4-(3-chloropropyl) piperazine (3) (0.0036 mol), 1,2,4-triazolo[4,3-a]pyridine-3-(2H)-one (4) (0.0115 mol).
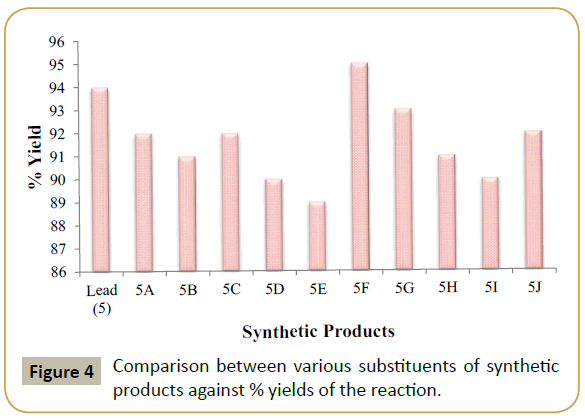
To explore the scope of our methodology, a wide variety of novel Trazodone analogues have been synthesized. The investigation results of which are summarized in (Table 2). Overall data indicated that, all products give admirable yields (88-95%) (Figure 4).
| Entry | Product | R1 | R2 | R3 | R4 | R5 | n | Yield (%)x | Melting Point (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Lead (5) | -H | -Cl | -H | -H | -H | 3 | 94 | 222 [223]a |
| 2 | 5A | -Cl | -Cl | -H | -H | -H | 3 | 92 | 253 [>250]a |
| 3 | 5B | -H | -Cl | -Cl | -H | -H | 3 | 91 | 251[>250]a |
| 4 | 5C | -H | -Br | -H | -H | -H | 3 | 92 | 211 [211-213]a |
| 5 | 5D | -H | -H | -F | -H | -H | 3 | 90 | 243 [240-242]a |
| 6 | 5E | -Cl | -H | -Cl | -Cl | -H | 3 | 89 | 252 [>250]a |
| 8 | 5F | 0 | -H | -H | 0 | -H | 3 | 95 | 232 [230-232]a |
| 9 | 5G | -C2H5 | -H | -H | -H | -H | 3 | 93 | 207 [204-206]a |
| 10 | 5H | -H | -Cl | -H | -H | -H | 2 | 91 | 219 [218-220]a |
| 11 | 5I | -H | -Cl | -H | -H | -H | 4 | 90 | 227 [227-230]a |
| 12 | 5J | Naphthyl | -H | -H | -H | 3 | 92 | 226 [225-227]a | |
Table 2 Synthesis of 2-[3-{4-(3-chlorophenyl)-1-piperazinyl} propyl]-1,2,4-triazolo[4,3-a]pyridine-3-(2H)-one hydrochloride (5) analogues. xYields refer to those of pure isolated products characterized by IR, 1H NMR and Mass spectra; aLambat and Deo [16].
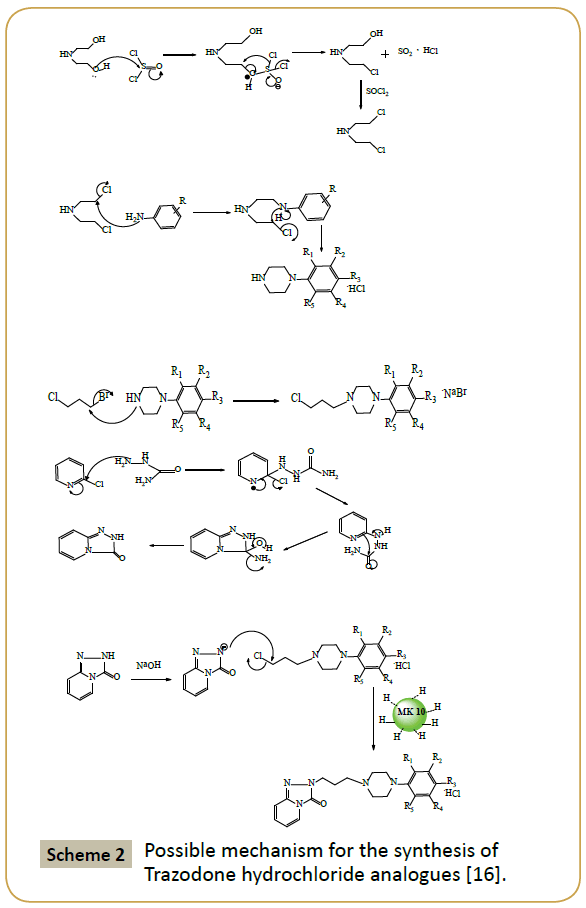
Several substituents such as chloro, fluoro, bromo, methoxy and ethoxy group were used to carry out the reactions. There was little or no effect of substituents as observed in compound 5F (Table 2) as far as the yield of product is concerned. It is because two electron donating methoxy groups were present on R1 and R4 of product (3) which makes the aromatic ring more electron rich. During the condensation reaction the chloro group present on the side chain of (3) was removed via SN2 displacement process and hence inversion products were expected in the reaction. The possible role of MK10 is depicted in the proposed mechanism (Scheme 2).
Scheme 2: Possible mechanism for the synthesis of Trazodone hydrochloride analogues [16].
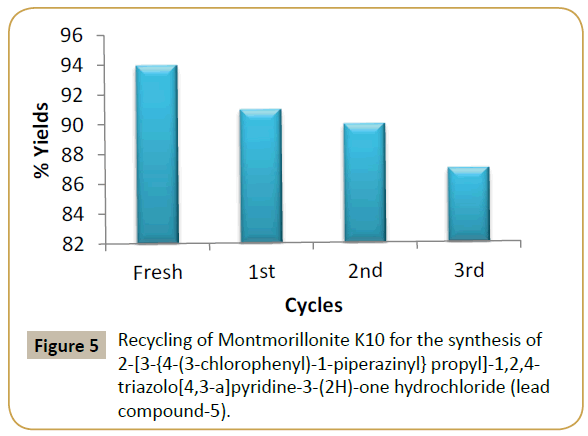
The recyclability potential of the MK10 catalyst was examined for the model reaction product of lead compound. The recycling procedure involved the separation of the catalyst from the reaction mixture simply by usual filtration. The recovered catalyst was purified by washing with ethyl acetate followed by drying in an oven. The results summarized in (Table 3) reveals that the catalyst has to be used for three successive times without loss of its activity. The reliability of the recovered catalyst was detected and proved to be as active as the new catalyst (Figure 5).
| Sr. no. | Cycle | Yield (%)x |
|---|---|---|
| 1 | Fresh | 94 |
| 2 | 1st | 91 |
| 3 | 2nd | 90 |
| 4 | 3rd | 87 |
Table 3 Recycling of MK 10 for the synthesis of model product (lead compound). XIsolated yields.
Conclusion
We have synthesized a novel extremely significant pharmacologically active scaffold by using MK10 catalyst, which proficiently activates the two component condensation for the production of (5) derivatives. The main advantages of the present synthetic methodologies are efficiency, versatility, good yield, short reaction times, cleaner reaction profile, convenient work-up, easy catalytic recyclability, and reusability with no loss of catalytic activity, which makes this protocol valuable and attractive in the improving of benign chemical processes and products.
Acknowledgements
The author Trimurti L. Lambat gratefully acknowledges the funding support rendered by the DST, New Delhi for the INSPIRE Fellowship [IF120418]. We thanks to the Director, Institute of Science, Nagpur for providing the necessary research facilities, SAIF Chandigarh for spectral analysis and Dr. Farhin S. Inam for the constant encouragement given.
References
- Bon RS, Vliet van B, Sprenkels NE, Schmitz RF, Kanter de FJJ, et al. (2005) Multicomponent synthesis of 2-imidazolines. J Org Chem 70: 3542-3546.
- Orru RVA, de Greef M (2003) Recent advances in solution-phase multicomponent methodology for the synthesis of heterocyclic compounds. Synthesis 10: 1471-1499.
- Hamid Reza S, Hossein Y, Majid G (2008) Silica supported perchloric acid (HClO4-SiO2): an efficient and recyclable heterogeneous catalyst for the one-pot synthesis of amidoalkylnaphthols. Tetrahedron 64: 1263-1269.
- Subbareddy K, Arumugam S, Brian CB (2001)Montmorillonite K10 catalyzed regioselective addition of Thiols and thiobenzoic acids onto olefins: an efficient synthesis of dithiocarboxylic esters. Tetrahedron Lett 42: 3791-3794.
- WieczerzakM, Namieśnik J, Kudłak B (2016) Bioassays as one of the Green Chemistry tools for assessing environmental quality: A review. Environ Int94: 341-361.
- Ramón DJ, Yus M (2005) Asymmetric Multicomponent Reactions (AMCRs): The New Frontier.Angew Chem Int Ed 44: 1602-1634.
- Ramachary DB, Barbas CFIII (2004) Cover Picture: Towards Organo-Click Chemistry: Development of Organocatalytic Multicomponent Reactions through Combinations of Aldol, Wittig, Knoevenagel, Michael, Diels-Alder and HuisgenCycloaddition Reactions.Chem Eur J 10: 5323-5331.
- Andreana PR, Liu CC, Schreiber SL (2004) Stereochemical Control of the Passerini Reaction. Org Lett6: 4231-4233.
- Burke MD, Schreiber SL (2004) A Planning Strategy for Diversity Oriented Synthesis. Angew Chem Int Ed 43: 46-58.
- Shanmugam P, Rajasingh P (2004) Studies on montmorillonite K10-microwave assisted isomerization of Baylis-Hillman adduct. Synthesis of E-trisubstituted alkenes and synthetic application to lignan core structures by vinyl radical cyclization. Tetrahedron 60:9283-9295.
- Pai NR, Dubhashi DS (2010) Studies of Antipsychotic drugs as potential schizophrenia agents. J Chem Pharm Res 2: 458-472.
- Tatsumi M, Groshan K, Blakely RD, Richelson E(1997) Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Euro J Pharmacol340: 249-58.
- Marek GJ, McDougle CJ, Price LH, Seiden LS (1992)A comparison of trazodone and fluoxetine: implications for a serotonergic mechanism of antidepressant action. Psychopharmacology (Berl) 109: 2-11.
- Lambat TL, Deo SS (2016) Synthesis of Novel Benzofluorenone Derivatives and their HIV-Reverse Transcriptase Inhibitory Activity.J Chin Adv Mat Soc.
- Lambat TL, Deo SS, Inam FS, Deshmukh TB, Bhat AR (2016)Montmorillonite K10: An efficient organo heterogeneous catalyst for one-pot synthesis of new N,N′-alkylidenebisamide derivatives under solvent free condition. Karbala Int J Mod Sci 2: 63-68.
- Lambat TL, Deo SS (2014) Sulphamic Acid: An Efficient and Green Synthesis of 2-[3-{4-(3-chlorophenyl)-1-piperazinyl} propyl]-1, 2, 4-triazolo [4, 3-a] pyridine-3- (2H)-one hydrochloride and its derivatives. Der Pharmacia Lett 6: 218-224.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences