ISSN : 2393-8854
Global Journal of Research and Review
Laboratory Information Systems and Analytical Turnaround Time
Rahad Zawawi1 and Taghreed Justinia2*
1King Saud Bin Abdulaziz for Health Sciences, College of Public Health and Health Informatics, Saudi Arabia
2IT Services Technology & Health Informatics, King Saud bin Abdulaziz University for Health Sciences, Saudi Arabia
- *Corresponding Author:
- Taghreed Justinia
PhD, Regional Director IT Services Technology & Health Informatics
King Saud bin Abdulaziz University for Health Sciences
College of Public Health & Health Informatics
Jeddah, Saudi Arabia
Tel: +96622245000
E-mail: JustiniaT@NGHA.MED.SA
Received date: January 09, 2017; Accepyed date: January 28, 2017; Published date: February 08, 2017
Citation: Zawawi R, Justinia T. Laboratory Information Systems and Analytical Turnaround Time. Glob J Res Rev. 2017, 4:1.
Abstract
Objective: The analytical turnaround time for laboratories is an indicator of their effectiveness and can be used as a quality indicator for laboratory medicine. It can also impact health care services. Laboratory information systems can impact the analytical turnaround time. Thus, any changes in these systems may affect the turnaround time for the laboratories they operate in. This study examines whether or not there is a significant improvement in the analytical turnaround time of the Haematology section at King Abdulaziz Medical City Laboratory Department in Jeddah after the implementation of the new laboratory information system.
Design: This is a one-group, pre-test – post-test, quasi-experimental study with no control group.
Results: Comparing the analytical turnaround time means of the tested panels between the new and the old laboratory information systems showed statistically significant differences. In general, a one-month increase was observed in the analytical turnaround time after the implementation of the new laboratory information system; however the analytical turnaround time was shown to have decreased after that the first month post implementation.
Conclusion: The analytical Turnaround Time of the Haematology section at the Laboratory Department of King Abdulaziz Medical City – Jeddah was shown to have improved after implementing the new Laboratory Information System. However, it took some time until the improvement occurred.
Keywords
Laboratory information systems, Turnaround time, Clinical laboratory, Health care
Introduction
Clinical laboratory plays a major part in aiding the health care providers to make accurate decisions, where it performs tests, which are requested by the health care providers on their patients' specimens and produce accurate and precise results [1]. These results must be available and accessible whenever they are needed by the healthcare providers [2].
It is very important for healthcare providers to obtain lab results as quick as possible in order to diagnose a medical condition or prescribe a medication in a timely manner [3,4]. This will lead to improve the efficiency and effectiveness of the delivered health care services, and as a result, the patients will be more satisfied. Conversely, patients' health condition may become worse in case those lab results are delayed [2]. It is also known that the faster the health care service provided, the better the outcome for the patient [5]. This means that timeliness, or in other words, fast turnaround time (TAT), is what health care providers desire for their requested lab tests, which will also improve their efficiency [6]. Moreover, there are times when they demand for fast TAT, especially for patients who need immediate medical care or continuous health condition monitoring [2]. They may even ready to accept results with low quality if this mean that they will receive them in shorter TAT [7]. For the Laboratory, the TAT is considered an indicator of its performance, which means the shorter the TAT the more efficient a laboratory is in producing reliable results in a timely manner [2-4,8]. TAT is also used as a quality indicator for laboratory medicine, in which it can help in evaluating the laboratory quality.
One of the serious worldwide problems is the overcrowding in the emergency department that can cause a delay in providing the required health care services when they are needed [9,10]. The laboratory can help in solving this problem by reducing the required TAT for emergency lab requests, where fast TAT results in improving the efficiency of the emergency department in addition to decrease the patient Length of Stay (LOS) in the emergency department [10]. In addition to that, it is mentioned in the literature that TAT is associated with patient LOS in general, where the shorter the TAT the shorter the LOS [2,11,12].
TAT in general has three phases, pre-analytical, analytical and post-analytical phases [4,8]. According to the literature, most of the laboratories are interpreting TAT as the time from receiving a sample in the laboratory until the time of result reporting (analytical phase). However, most of the clinicians are interpreting it as the time from ordering a test until receiving its result (i.e. Total TAT which includes pre-, post- and analytical phases) [2,3,11]. Therapeutic TAT is another term for TAT that starts from physician's test ordering until making of treatment decision based on the test result [3,4].
Different tests have been chosen by different studies in order to measure the laboratory TAT (i.e. analytical TAT). Bilirubin for example was chosen in one study, because of being a common test in the studied hospital [8], while complete blood count (CBC) and basic metabolic panels were chosen in another, based on the centrifugation needs [6]. While in one of the Q-probe studies of the College of American Pathologist, CBC and basic metabolic panels in addition to Thyroid Stimulating Hormone tests have been used because they are the most commonly ordered tests in the hospitals that have been involved in their study [13]. These studies made it clear that the most commonly ordered tests are the ones that should be included in the evaluation of the analytical TAT to gain accurate and reliable results.
Laboratory information system (LIS) plays a very important role in clinical laboratories’ operation, where it is responsible for the utilization and archiving of laboratory tests’ results [14]. According to the literature, implementing LIS in clinical laboratories improved their analytical TAT [15]. Furthermore the LIS plays an important role in improving the TAT and overall quality of healthcare, where it allows the communication between the analyzers and the hospital information system, which increases result's quality and decreases human errors [16]. In other words, it has the capability to improve the post analytical phase of the TAT as well [17]. In addition, there is a study, which showed that there is a significant positive association between the availability of an LIS in a hospital and its revenue whether in inpatient or outpatient settings [17].
There are a number of studies in the literature that evaluate the analytical TAT of their current LIS [2,4,18]. However, the aim of this study is different, which is to answer the following question: Has the implementation of the new Laboratory Information System improved the Analytical Turnaround Time of the Haematology section at King Abdulaziz Medical City (KAMC) Laboratory Department in Jeddah? In order to answer this question, the data regarding analytical TATs of some test panels before and after the implementation of the new LIS in the mentioned section have been analyzed. In a similar study, Lowe, Griffin & Hart conducted an analysis on their analytical TATs before and after the conversion of their entire hospital information system [19]. However, they found that the difference in the analytical TAT between their old and new hospital information system is not significant.
To summarize, it is the patient's right to get proper healthcare services in a timely manner. Therefore, health care providers need to get the results of the requested Lab tests in a short TAT in order to treat and diagnose patients properly. This means that the hospital laboratory department needs to improve its performance by shortening its analytical TAT, and LIS can play an important role in doing so. Evaluating analytical TAT has been the aim of a number of studies, even though, every hospital is different in terms of LISs, instrumentations, settings, workflow, patients… etc. In addition, different studies use different tests. Thus, every study is different from the other. The purpose of this study is to determine whether the new LIS has improved the Analytical TAT of the Haematology section at KAMC Laboratory Department in Jeddah or not.
Method
Study design
The study design involved cause and effect, in other words, it is a quasi-experimental study, where one - group pretest - posttest method have been used with no control group, where the intervention in this study was the new LIS, which has been in use at KAMC – Jeddah since November, 2012 replacing the old LIS.
Study setting
Study area: The study has been conducted in the Haematology section of the Laboratory at KAMC – Jeddah. KAMC - Jeddah was founded in July 1982. It provides many health care services in different specialties, which are directed mainly toward National Guard employees and their dependents. In addition, it serves both inpatients and outpatients [20]. The Laboratory and Pathology Department in KAMC – Jeddah is one of the critical departments that provide health care services. Haematology section is one of the important sections within the department, which perform many tests that significantly aid health care providers in making their diagnoses regarding their patients' health condition. It has been accredited by the College of American Pathologist [21].
Study’s systems: The old Laboratory Information System, which was used in KAMC - Jeddah, had a text-based user interface, where users could only use the keyboard to enter sample numbers, enter and view results, navigate between applications and to perform other actions. It had few applications that the laboratory technologist could utilize. In addition to that, not all of the laboratories instruments were interfaced to it. The system could only hold 3 months of patients' results in order to function normally, and the rest of the results had to be archived in external storing media. This means that users, including health care providers, had a direct access to only 3 months of lab results, and if older information were required, the user needed to contact the LIS administrator in order to retrieve it for them for limited time.
Generally speaking, legacy systems have been known to have several drawbacks. These drawbacks include but not limited to being operated using obsolete hardware, which is costly to maintain and slow as well. In general, the expense of maintaining its software is high. Another drawback is the difficulty to integrate it to other applications or system, which caused by the unavailability of interfaces with high capability. Lack of knowledge about how these systems work from the inside, in addition to the absence of documentation, make it expensive and time consuming to track problems a. Another drawback is that legacy systems are very difficult to be upgraded [22].
The new LIS in KAMC - Jeddah has more advanced features. It has a graphical user interface. In addition, users can use the mouse, keyboard and a barcode reader to perform different actions. It has many applications to be utilized by users. In addition, the majority of the laboratory's instruments became fully interfaced to it. This new system does not have a limitation in storing patients' results, and therefore, users have direct access to all patients' lab results. Even though new LIS has more application and functionalities, it is not known whether it can improve the lab’s analytical TAT or not. Therefore, the performance of the new LIS needed to be assessed.
Systems’ hardware: There was an upgrade of the hardware to meet the recommended requirements for the new system to function as it was designed to. However, the network infrastructure remained the same. The hardware of the old LIS was mainframe based, which was accessed by the users through client software that had been installed in the lab computers. However, the new LIS will not work on the same hardware, therefore, it was necessary to upgrade them. The new LIS is operated using server based hardware, and the users access the system through a web interface. Although the hardware was upgraded, the purpose of this study is not to compare the hardware or software specifications between the two systems. The focus is on the overall performance of the lab after the implementation of the new LIS, with the analytical TAT being an indicator.
The hardware upgrade did not affect the outcome of the study. To demonstrate this, the manner in which the analytical TAT is calculated needs to be explained. Analytical TAT is the outcome of subtracting the testing panel’s receiving time from its verifying time [2]. Once the sample arrived in the laboratory, the lab technologist registers it in the system, which is recorded as the receiving time. The lab technologist then performs the requested testing panel using a specific lab instrument. After getting the results, they are then validated and verified. The time of verifying the results is recorded in the system as the verifying time. This means that the hardware does not impact calculating the analytical TAT. Therefore, the upgraded hardware had no effect on the study.
Selection and description of study subjects
There are many tests that are performed in the hematology section of the Laboratory at KAMC – Jeddah. These include but not limited to Body Fluid Cell Count, Blood Smear, CBC and Differential panel, Coagulation Panel, D Dimer, Erythrocyte Sedimentation Rate, Fibrinogen, Malaria Smear, Sickle Cell Solubility Test, Von Wiliebrand Factor Antigen… etc. CBC and Differential panel, in addition to Coagulation panel, represent about fifty percent of the performed tests. In the other hand, the section is using a number of different priorities, where each test has a priority assigned to it. These priorities include but are not limited to Routine, Timed Study, STAT (Short TAT)… etc. where Routine priority is assigned to about 58% of the tests, and STAT priority is assigned to about 36% of the tests. These figures show that CBC and Differential, and Coagulation panels, in addition to STAT and Routine priorities, represent the greater percentage of the total workload in the hematology section of the Laboratory at KAMC – Jeddah. Therefore, they are the targeted population that were included in the study and other panels and priorities have been excluded.
The analytical TAT of all Coagulation and CBC and Differential panels, with either Routine or STAT priority, that have been performed in the last 17 months before the implementation of new LIS and in the first 17 months after the implementation have been included in this study. Data regarding the analytical TAT for the panels that have been performed before the new LIS implementation have been extracted from the old LIS database. In the other hand, for those that have been performed after the implementation, their analytical TAT data have been extracted from the new LIS database.
Ethical considerations
This study involved the collection of analytical TAT data for already verified patients’ testing panels, and no observation or interviews have been conducted. TAT data are stored in the new and old LISs’ databases, which are under the responsibility of the researcher's department. Therefore, permission for using these data has been obtained from the researcher’s department. Furthermore, in order to make sure that patients’ security and confidentiality are not affected, all patients identifiers have been removed from the collected data. The targeted population has been all included in the study, and therefore, the possibility of sampling bias has been eliminated. In addition, the study has been approved by the Institutional Review Board of King Abdullah International Medical Research Center. The protocol number of the study is SP14/155 (See Appendix for the approval letter).
Data collection
In KAMC - Jeddah, a new LIS, which replaced the old LIS, went live in November, 2012. Data regarding the analytical TATs (dependent variable) for Coagulation and CBC and Differential test panels with either STAT or Routine priorities (independent variable 1) have been gathered from the old LIS and the new LIS (independent variable 2). Analytical TAT data has been collected from the old LIS database for a period of 17 months before the go-live of the new LIS. Also, the analytical TAT data for the same test panels have been gathered from the database of the new LIS for a period of 17 months after the go-live. Table 1 provides a summary of all variables, their operational definitions, collection methods and measurement in addition to their types. There will be no control group in this study.
| Variable | Definition | Method | Measure | Type |
|---|---|---|---|---|
| IDV1 Test Panel and Testing Priority | A test's panel is composed of a group of tests which are related to each other. And priority is how soon the result should be verified. | Data have been extracted from LIS database. | There have been four categories: STAT Complete Blood count and Differential Panel, Routine Complete Blood count and Differential Panel, STAT Coagulation Panel, and Routine Coagulation Panel. | Nominal |
| IDV2 LIS | An LIS is a system that has many applications which handle information generated by medical laboratory processes. | From which database the TAT have been extracted. | There have been two LISs, the old LIS and the new LIS | Nominal |
| DV Turnaround time | For this study the analytical turnaround time (TAT) will be used, which is the period of time from receiving the blood sample in the section until the verification of its tests results. | Data regarding analytical TAT have been extracted from LIS database. | The analytical TAT have been measured in the following format: | Interval |
Table 1: Study variables.
Instrument validity and reliability: Analytical TAT for test X is the period between the time of receiving the patient specimen with test X request, until the time of verifying the result of test X for the same patient specimen. These two points of time are automatically recorded in the database of the used LIS. For the new LIS, the analytical TAT is calculated by the system itself, which is validated by the laboratory for official use. Every time the analytical TAT for test X is requested from the new LIS, the result will be the same. Therefore, the new LIS produce valid and reliable analytical TAT. After the implementation of the new LIS, all of the old LIS data have been migrated into a Microsoft Access database, which has been validated by the hospital’s Information Services Department. In order to calculate the analytical TAT for test Y, which has been done using the old LIS, a process of two steps need to be performed. The first step is to extract the times of receiving the patient specimen with test Y request and the verifying of its result from the old LIS database using Microsoft Access software. The second step is to import the extracted times into Microsoft Excel software and then use a simple formula to subtract the receiving time from the verifying time, and the result will be the analytical TAT for test Y. In this study, the extraction and the calculation steps have been performed using Microsoft Access 2013 and Microsoft Excel 2013 respectively. Repeating the two steps for the same test will produce the same analytical TAT every time. Therefore, analytical TATs that have been obtained from the old LIS using the above extraction and calculation steps are reliable and valid.
Study internal and external validity: In the study, the used subjects were different testing panels (i.e. CBC and Differential, and Coagulation Panels) with different priorities, where each testing panel have been received and verified in the same day within a specific time frame. Testing Panels, which have been tested before the implementation of the new LIS were different as individuals from those that have been tested after the implementation, even though, they both had same requirements. Also, all CBC and Differential, and Coagulation panels in the study period, whether before or after the implementation, have been included in the study. Therefore, there are no maturation, testing, selection or interaction threats to the internal validity of this study
The majority of tests that have been performed in the Haematology Lab at KAMC – Jeddah are CBC and Differential, and Coagulation panels, and the most used test priorities are either routine or STAT. The results of the study have represented the targeted population since they all have been included. Regarding the generalizability to other populations, this other ones should have similar setting to this study (i.e. the same panels and their component, test measurement methodologies, the same old and new LISs and similar workflow) in order for this study to be generalizable to them. In other words, this study has a low generalizability to other populations.
Data analysis
To find if there is a significant difference in the analytical TAT between the old LIS (i.e. before the go-live) and the new LIS (i.e. after the go-live), the Statistical Package for Social Science (SPSS) program have been used to enter the collected data for all variables, then coded and labelled in order to do the data analysis. To answer the study question, a comparison has been conducted between the mean of the Routine Panels’ analytical TAT for the old LIS and the mean of the same Panels for the new LIS, as well as between the mean of the STAT Panels’ analytical TAT for the old LIS and the mean of the ones for the new LIS. In addition, detailed comparisons have been conducted for each category, where the means of the analytical TAT have been compared between the old LIS and the new LIS for Routine CBC and Differential Panel, STAT CBC and Differential Panel, Routine Coagulation Panel as well as for STAT Coagulation Panel. The comparisons have been conducted using the independent t test as a statistical tool with alpha of 0.05 as the significance level. In addition, in order to compare the performance of the haematology section for the studied panels before and after the implementation of the new LIS, a time series analysis has been conducted.
Results
The analytical TAT data of the testing panels’ has been collected for a period of 34 months in total. The number of the extracted records in the first 17 months from the old LIS database was 266055. For the next 17 months it was 267770, which has been extracted from the new LIS. Comparing the analytical TAT means for the Routine panels between the old and the new LIS using independent t test gave a P value of less than 0.001 (t=3.859) (Table 2), where the mean for the old LIS was 35 minutes with a standard deviation (SD) of eight minutes, and for the new LIS it was 26 minutes with a SD of nine minutes (Table 3). For the STAT panels, the comparison yielded a P value of 0.033 (t=2.178) (Table 4), where the mean for the old LIS was 30 minutes with a standard deviation (SD) of eleven minutes, and for the new LIS it was 25 minutes with a SD of ten minutes (Table 5).
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 1.587 | 0.212 | 3.859 | 66 | 0 | 00:08 | 00:02 | 00:04 | 00:13 |
| Equal variances not assumed | 3.859 | 65.297 | 0 | 00:08 | 00:02 | 00:04 | 00:13 | |||
Table 2: Independent t test for routine panels.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 34 | 00:35 | 00:08 | 00:01 |
| New | 34 | 00:26 | 00:09 | 00:01 |
Table 3: Descriptive statistics for routine panels.
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 6.76 | 0.011 | 2.178 | 66 | 0.033 | 00:05 | 00:02 | 00:00 | 00:10 |
| Equal variances not assumed | 2.178 | 65.318 | 0.033 | 00:05 | 00:02 | 00:00 | 00:10 | |||
Table 4: Independent t test for STAT panels.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 34 | 00:30 | 00:11 | 00:01 |
| New | 34 | 00:25 | 00:10 | 00:01 |
Table 5: Descriptive statistics for STAT panels.
Furthermore, P values of less than 0.001 have been obtained by comparing the analytical TAT means between the Old and the new LISs for each panel. Detailed results can be found on Tables 6-9. For CBC and Differential Panel, the routine panel had a mean of 26 minutes (SD=2) for the Old LIS, while it was 18 minutes (SD=6) for the new LIS (Table 10). The means for the STAT CBC and Differential Panel were 19 minutes (SD=1) and 15 minutes (SD=3) for the old LIS and the new LIS respectively (Table 11). In case of Routine Coagulation Panel, the old LIS and the new LIS had means of 43 minutes (SD=2) and 34 minutes (SD=3) respectively (Table 12). While for the STAT panel, they were 41 minutes (SD=1) for the old LIS and 34 minutes (SD=2) for the new LIS (Table 13).
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 2.033 | 0.164 | 5.406 | 32 | 0 | 00:08 | 00:01 | 00:05 | 00:11 |
| Equal variances not assumed | 5.406 | 19.582 | 0 | 00:08 | 00:01 | 00:05 | 00:11 | |||
Table 6: Independent t test for routine CBC and differential panel.
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 3.19 | 0.084 | 4.621 | 32 | 0 | 00:04 | 00:00 | 00:02 | 00:05 |
| Equal variances not assumed | 4.621 | 18.966 | 0 | 00:04 | 00:00 | 00:02 | 00:05 | |||
Table 7: Independent t test for routine STAT CBC and differential panel.
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 0.346 | 0.561 | 9.619 | 32 | 0 | 00:08 | 00:00 | 00:06 | 00:10 |
| Equal variances not assumed | 9.619 | 27.421 | 0 | 00:08 | 00:00 | 00:06 | 00:10 | |||
Table 8: Independent t Test for routine coagulation panel.
| Levene's Test for Equality of Variances | t-test for Equality of Means | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | Sig. | t | df | Sig. (2-tailed) | Mean Difference | Std. Error Difference | 95% Confidence Interval of the Difference | |||
| Lower | Upper | |||||||||
| ATAT | Equal variances assumed | 2.444 | 0.128 | 8.814 | 32 | 0 | 00:07 | 00:00 | 00:05 | 00:08 |
| Equal variances not assumed | 8.814 | 26.67 | 0 | 00:07 | 00:00 | 00:05 | 00:08 | |||
Table 9: Independent t test for STAT coagulation panel.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 17 | 00:26 | 00:02 | 00:00 |
| New | 17 | 00:18 | 00:06 | 00:01 |
Table 10: Descriptive statistics for routine CBC and differential panel.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 17 | 00:19 | 00:01 | 00:00 |
| New | 17 | 00:15 | 00:03 | 00:00 |
Table 11: Descriptive statistics for STAT CBC and differential panel.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 17 | 00:43 | 00:02 | 00:00 |
| New | 17 | 00:34 | 00:03 | 00:00 |
Table 12: Descriptive statistics for routine coagulation panel.
| LIS | N | Mean | Std. Deviation | Std. Error Mean | |
|---|---|---|---|---|---|
| ATAT | Old | 17 | 00:41 | 00:01 | 00:00 |
| New | 17 | 00:34 | 00:02 | 00:00 |
Table 13: Descriptive Statistics for STAT coagulation panel.
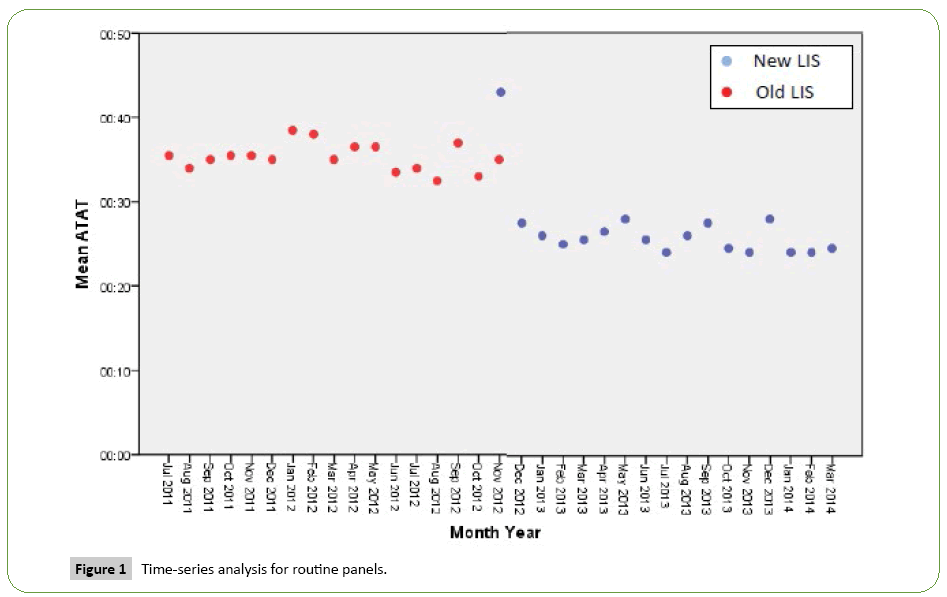
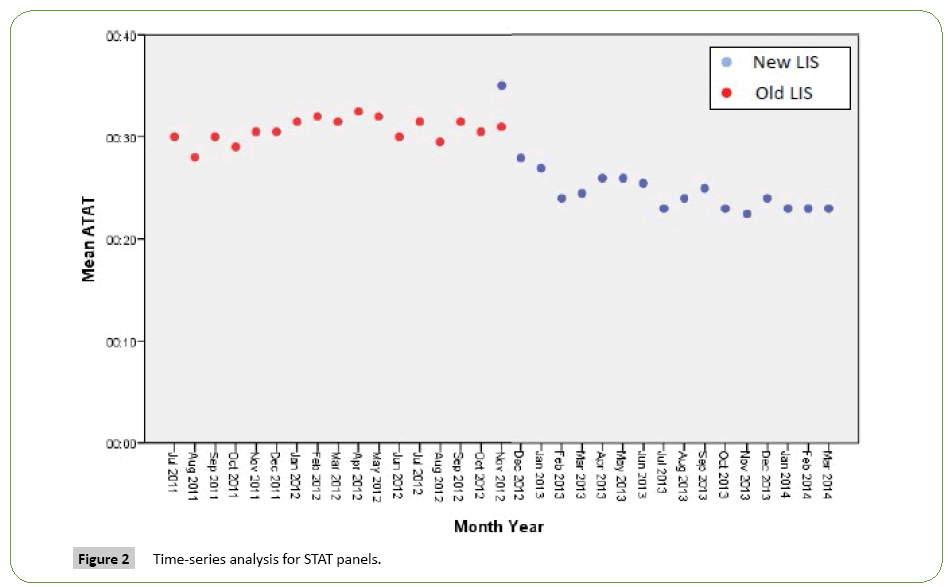
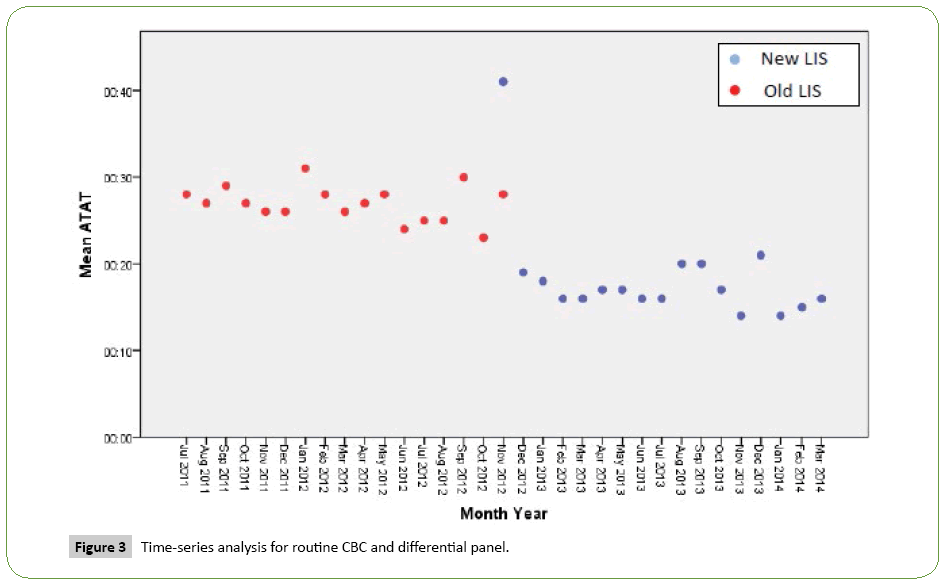
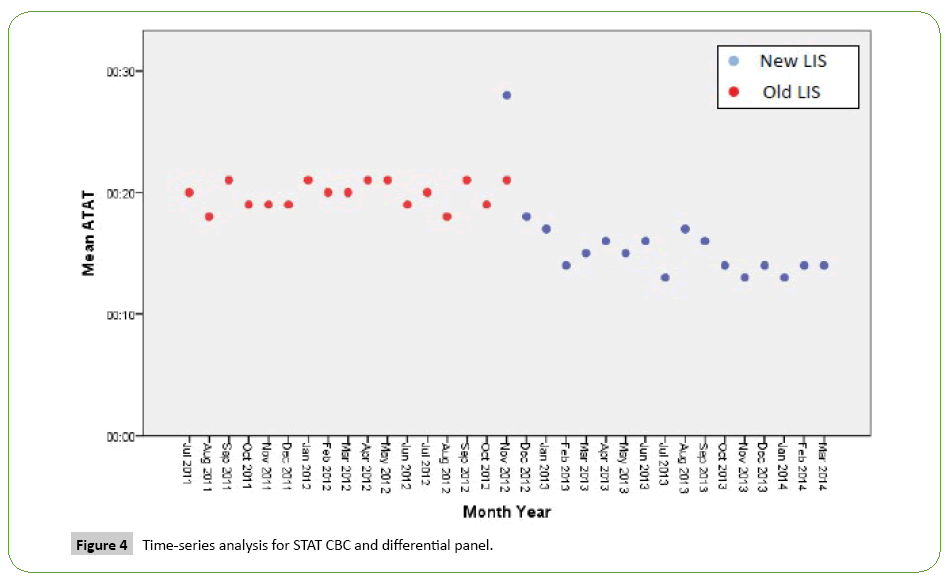
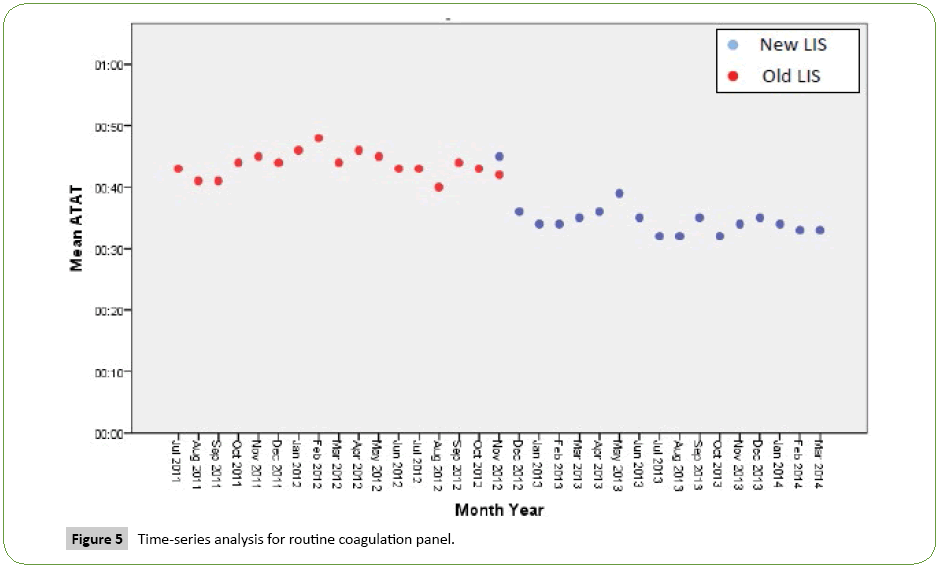
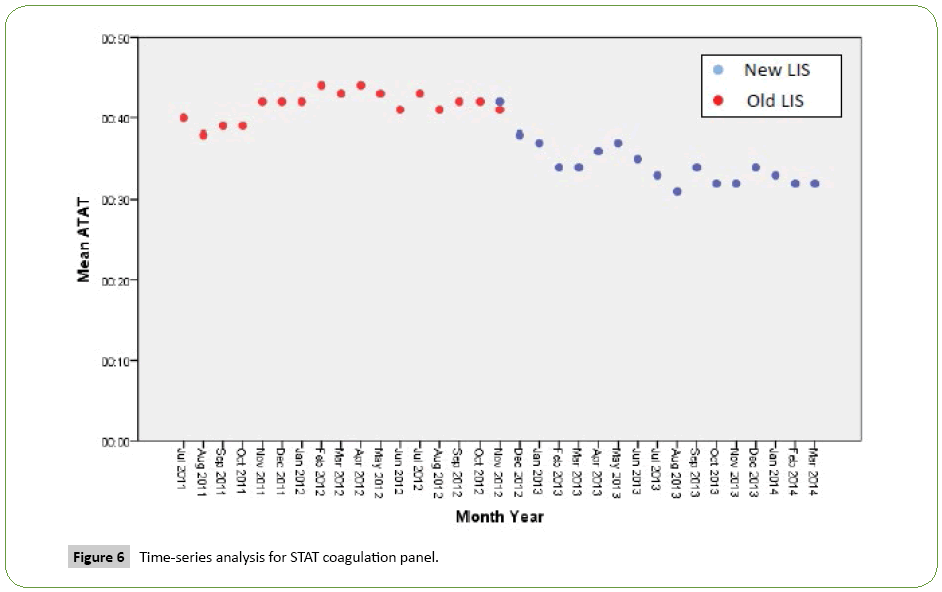
Time series analysis for the analytical TAT of the Routine Panels in general showed an increase in the mean of the first month after the implementation of the new LIS, however, the analytical TAT means of the following months became lower than analytical TAT means for the months before the implementation (Figure 1). For the STAT Panels in general, the analytical TAT mean has also increased in the first month after the implementation, and in the second month it returned to a similar level to the means of the months before the implementation. It only became lower starting from the third month (Figure 2). Similar results have been seen after doing a time series analysis for the analytical TAT of both Routine CBC and Differential Panel and STAT CBC and Differential Panel. The mean for the Routine CBC and Differential Panel has increased in the first month after the implementation, and started to become lower from the second month (Figure 3), and for the mean of the STAT CBC and Differential Panel, it has also increased in the first month after conversion, and started to become lower from the third month onward (Figure 4). However, the mean of the analytical TAT of both Routine and STAT Coagulation Panels did not increase in the first month after the implementation, but it started to become lower from the second month after the implementation for the Routine Coagulation Panel comparing to the months before the implementation (Figures 5 and 6). While For the STAT Coagulation Panel, the analytical TAT mean started to become lower from the fourth month after the implementation.
Discussion
The study showed that there is a statistically significant difference between the mean of the old LIS analytical TAT and the mean of the new LIS analytical TAT (P<0.001) for all panels. There was an improvement of 31% (8 minutes), 21% (4 minutes), 21% (9 minutes) and 17% (7 minutes) for Routine CBC and Differential Panel, STAT CBC and Differential Panel, Routine Coagulation Panel and STAT Coagulation Panel respectively. In general, the analytical TAT means for both STAT and Routine panels have been improved after implementing the new LIS (P<0.001). The new LIS provides a number of applications and functionality that were not available in the old LIS. These new applications and functionalities have may helped the users in the Haematology section to utilize their work time better than before. However, the improvement has started from the second month after the implementation for the Routine Panels, while for the STAT Panels the improvement has started from the third month after the implementation.
There are not enough studies about the improvement in the analytical TAT after converting from one LIS to another. However, the study that has been done by Lowe showed that their analytical TAT did not significantly improve after implementing their new LIS [19]. In the contrary, this study showed that the new LIS has significantly improved the analytical TAT for both Routine (P<0.001, t=3.859) and STAT (P=0.033, t=2.178). However, the improvement was about 26% for Routine Panels and 17% for STAT panels. This information is important for the laboratory administration if improving the analytical TAT was one of their goals for converting to a new LIS. If it was a goal to be achieved, they have to consider the percentage of improvement whether it meet their expectation or not.
A study by Prijatelj showed that that implementing an LIS in their clinical laboratory, which was using a manual, paperbased system, has improved their analytical TAT by 25%, where all of their tests were treated as STAT tests [15]. In this study, converting from an old LIS to a new LIS have improved the laboratory’s analytical TAT for the STAT Panels by 17%, which is not that far from the results that obtained by Prijatelj and his colleagues.
In the study that was performed by Lowe et al. [19], their analytical TAT has increased for 5 months after the implementation of their new LIS, and then returned to the pre implementation level and stayed so until the end of the eighth month. Starting from the ninth month, their analytical TAT showed some improvement, but it was not a significant improvement comparing to their analytical TAT from the pre implementation period [19]. However, this study had slightly different results, where the analytical TAT has increased in the first month after the implementation of the new LIS only for both Routine and STAT Panels. While it has become significantly improved starting from the second month after the implementation for the Routine Panels, and it from the third month in case of the STAT Panels.
According to Lowe et al., the increase in analytical TAT in the first five months after implementing their new LIS had several reasons. One of the reasons was that their new LIS needed to be reset several for fixes and other issue, making the staff to wok manually using paper-based system. In addition, the new LIS was challenging to the laboratory staff, which took them long time to get used to it. Another issue was that lab requests were not order the way they should, which required the laboratory staff to communicate with the person who placed the order and ask him to complete the missing information. However, the analytical TAT for this study was only increased in the first month after the implementation of the new LIS. This means that the staff were adequately trained, and this increase occurred because they needed to adapt to the new LIS.
Using a census instead of a sample in a research, will eliminate any sampling error, and yield a result that truly represents the studied population [23]. In this study, every panel from the targeted population has been included. Therefore, the results that have been obtained represent the true improvement values for the STAT and Routine panels. The same approach has been used in many TAT involved studies [2,4,10,18]. Even though, population sampling approach has been also used [8,19].
Study Limitations
The mean of the analytical TAT has been used as the statistical tool for the comparison. Other statistical tools may produce different results.
Future work
The study was conducted only for the Haematology section, which is one of many sections in the Laboratory Department. Similar study can be conduct for the other sections as well. In addition, the new LIS has many applications and features, which also can be studied to know their specific effect on the analytical TAT of the deferent Laboratory sections. Moreover, other phase of TAT, including the pre and the post-analytical TAT can be studied as well.
Conclusion
The study proved that the implementation of the new Laboratory Information System has improved the analytical Turnaround Time of the haematology section at King Abdulaziz Medical City in Jeddah. However, the improvement did not occur directly after the implementation, where it took a month in case of the Routine Panels and two months in case of the STAT Panels in order to show any improvement.
Summary points
Known
• The different types of TAT
• LIS is associated with TAT improvement
Added to our knowledge
• Upgrading LIS can improve the laboratory analytical TAT to a certain percentage.
• Adequate staff training greatly reduces post LIS conversion issues.
Authorship
Mr. Zawawi contributed to the study concept, design of the study, analysis, interpreting the findings, and writing of the manuscript. Dr. Justinia was responsible of supervising the research, reviewing the draft, and approving the final version of the manuscript.
Acknowledgment
I'd like to thank Dr. Taghreed Justinia for her continuous support through the project. Many thanks to Ms. Al Dabbagh for the many discussions and valuable comments. A special thanks to my mother, father and my wife, who always motivate me and push me toward success.
Conflict of Interest
No conflict of interest.
References
- Barth JH (2012) Clinical quality indicators in laboratory medicine. Annals of clinical biochemistry 49: 9-16.
- Chien TI, Lu JY, Kao JT, Cheng YC, Lee YF (2007) Evaluation and improvement strategy of analytical turnaround time in the stat laboratory. Journal of the Formosan Medical Association 106: 558-564.
- Dey B, Bharti JN, Chakraborty M (2013) Laboratory Turnaround Time. International Journal of Health Sciences and Research 3: 82-84.
- Goswami B, Singh B, Chawla R, Gupta V, Mallika V (2010) Turn Around Time (TAT) as a Benchmark of Laboratory Performance. Indian Journal of Clinical Biochemistry 25: 376-379.
- Holland LL, Smith LL, Blick KE (2005) Reducing Laboratory Turnaround Time Outliers Can Reduce Emergency Department Patient Length of Stay An 11-Hospital Study. American journal of clinical pathology 124: 672-674.
- Stotler BA, Kratz A (2012) Determination of Turnaround Time in the Clinical Laboratory “Accessioning-to-Result” Time Does Not Always Accurately Reflect Laboratory Performance. American journal of clinical pathology 138: 724-729.
- Hawkins RC (2007) Laboratory turnaround time. The Clinical Biochemist Reviews 28: 179.
- Elhoseeny T, Mohammad E (2013) Quality of the clinical laboratory department in a specialized hospital in Alexandria. Egypt, EMHJ 19: 81-87.
- Forster AJ, Stiell I, Wells G, Lee AJ, Van Walraven C (2003) The effect of hospital occupancy on emergency department length of stay and patient disposition. Academic Emergency Medicine 10: 127-133.
- Storrow AB, Zhou C, Gaddis G, Han JH, Miller K, et al. (2008) Decreasing lab turnaround time improves emergency department throughput and decreases emergency medical services diversion: a simulation model. Academic Emergency Medicine 15: 1130-1135.
- Shahangian S, Snyder SR (2009) Laboratory Medicine Quality Indicators A Review of the Literature. American journal of clinical pathology 131: 418-431.
- Lee EJ, Shin SD, Song KJ, Kim SC, Cho JS, et al. (2011) A point-of-care chemistry test for reduction of turnaround and clinical decision time. The American journal of emergency medicine 29: 489-495.
- Valenstein P, Walsh M (2003) Five-year follow-up of routine outpatient test turnaround time: a College of American Pathologists Q-Probes study. Archives of pathology & laboratory medicine 127: 1421-1423.
- Sepulveda JL, Young DS (2013) The ideal laboratory information system. Archives of Pathology and Laboratory Medicine 137: 1129-1140.
- Prijatelj V, Vuckovic A, Cerne D (1999) The optimization of turnaround time for blood samples in an emergency clinical laboratory. Studies in health technology and informatics pp: 610-616.
- Harrison JP, McDowell GM (2008) The role of laboratory information systems in healthcare quality improvement. International Journal of Health Care Quality Assurance 21: 679-691.
- Park WS, Yi SY, Kim SA, Song JS, Kwak YH (2005) Association between the implementation of a laboratory information system and the revenue of a general hospital. Archives of pathology & laboratory medicine 129: 766-771.
- Chung HJ, Lee W, Chun S, Park HI, Min WK (2009) Analysis of turnaround time by subdividing three phases for outpatient chemistry specimens. Annals of Clinical & Laboratory Science 39: 144-149.
- Lowe GR, Griffin Y, Hart M (2013) Analysis of Stat Laboratory Turn-Around-Times Before and After Conversion of the Hospital Information System. Respir care 59: 1275-1280.
- King Abdulaziz Medical City-Jeddah (2013) Kingdom of Saudi Arabia, Ministry of National Guard Health Affairs, Saudi Arabia.
- Laboratory and Pathology Department at KAMC – Jeddah
- Bisbal J, Lawless D, Wu B, Grimson J, Wade V, et al. (1997) An overview of legacy information system migration. Software Engineering Conference, APSEC'97 and ICSC'97 Proceedings pp. 529-530.
- Census and sample (2013) Australian Bureau of Statistics.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences