Investigating Mutations in Member 6 (ABCC6) gene, of Sub-family C (CFTR/MRP) of the ATP-binding Cassette Causing Pseudoxanthoma Elasticum
Sharika Akhter1, Thomas Georgas2, Georgios Giannoukos3*
1University of Greenwich, London, UK
2Saint Francis Xavier College , London , UK,
- *Corresponding Author:
- Georgios Giannoukos
Second Chance School, Greece.
Tel: 6972147019
E-mail: g.giannoukos@gmail.com
Received date: August 17, 2018; Accepted date: August 28, 2018; Published date: August 31, 2018
Citation: Akhter S, Georgas T, Giannoukos G (2018) Investigating Mutations in Member 6 (ABCC6) gene, of Sub-Family C (CFTR/ MRP) of the ATP-binding Cassette Causing Pseudoxanthoma elasticum. J Biol Med Res. Vol.2 No.2:17.
Abstract
Mutations in the ATP-Binding Cassette subfamily C number 6 gene (ABCC6) gene causes Pseudoxanthoma elasticum (PXE), a rare genetic disorder, typically inherited in an autosomal recessive manner. ABCC6 gene is a member of ATP-Binding Cassette (ABC) transporter family. ABC Transporter family proteins are ATP- dependent pumps hence they are named ATP-binding cassette (ABC) transporter family. This paper examines existing literature on mutations of the specific gene, identifies the mechanisms causing PXE and offers a detailed overlook on the progress made in investigating different mutations of the gene. This study also discusses expression of PXE despite of not inheriting PXE causing mutation but because of a primary abnormality as β-thalassemia a haemoglobinopathy condition, receiving unscrewed liver transplant from a PXE patient or severe reaction to medicine such as Penicillamine.
Keywords
ABCC6 gene; Sub-family C; ATP-binding cassette; Pseudoxanthoma elasticum
Introduction
The ABCC6 gene was discovered in 2000 (Terry 2013) by the scientist Sharon F. Terry and her husband Patrick F. Terry with the aid of colleagues of various health professions. Mutations in the ATP-Binding Cassette subfamily C number 6 gene (ABCC6) gene cause Pseudoxanthoma elasticum (PXE). This further led them to discovering certain mutations in the ABCC6 gene that results in PXE [1-3]. Terrys’ quest for investigating the disease began from noticing peculiar dermatological symptoms displayed by their children. Dermatologist Lionel Bercovitch made the diagnosis of PXE after examining their children. PXE is a rare genetic disorder, typically inherited in an autosomal recessive manner. Prevalence of PXE is believed to be 1 in 25000. An autosomal dominant genotype can also be inherited and happens to be very rare compared to autosomal recessive inheritance [4]. PXE can also be required through organ transplants from individuals with PXE, have an inherited condition of B- thalassemia or due to reaction to penicillamine medication [5,6]. It is also classed as a multisystem disorder as the disease involves the impairment of several organs and tissues. Finger et al. showed that the tissues and organ typically affected are the eyes, flexor skin regions such as the neck and armpits, connective tissue such as the inside of the lower lip, tissue of the vascular walls. Impairment of these organs and tissue manifest dermatological conditions around the flexor skin regions, vision disorders, and cardiovascular abnormalities. Ectopic mineralization and fragmentation of elastic fibers in connective tissues, skin and vascular walls is the main cause behind the impairment of these organs [4]. The possible reason(s) behind such abnormal mineralization in abnormal places remain unclear [4]. PXE is predominantly caused by mutations in the ATP-Binding Cassette subfamily C number 6 gene (ABCC6) genes. ABCC6 is a member of the ABC transporter protein family which plays several essential transporting roles including transportation of endogenous substrates, drugs, lipids, proteins, sugars, metal ions and compounds alike [7]. What endogenous substrate is transported by the ABCC6 protein is still a mystery which indicates that there is a need for more intensive investigations and researches to discover the underlying defect and what effect does it have on the substrate that is being transported [4].
During thorough researching, Sharon and Patrick F. Terry struggled to find literatures offering detailed information about the incidence of the disease, cause of the disease and possible treatments available for the disease, all of which were very poorly available. After having researched for a significant length of time, they realized that enough investigations were not performed for the disease and the gene responsible for controlling the disease was not yet discovered [1].
It was then when they started constructing a cohort of people who were closely connected to PXE i.e., Health professional careers, medical researchers or individuals with personal connections i.e., an affected family member and started collaborating to investigate as much as possible about the disease [1].
Literature Review
Mode of inheritance of PXE
In most of the cases PXE appears to be sporadic with apparently no family history of PXE. Although PXE occurs sporadically, there are two main ways of inheriting the disease: autosomal recessive and autosomal dominant of which autosomal recessive is the most common occurrence. On the sole basis of phenotype, 2 forms of both autosomal recessive and autosomal dominant PXE were proposed by Pope [8,9]. A third form autosomal recessive with peculiar combination of clinical manifestations; very mild skin lesions and severe vision abnormalities may potentially exist, which was recognized among 64 patients of South Africa and Zimbabwe origin.
Acquired PXE
Although severe liver abnormality is not heard of in PXE patients, a study by Bercovitch et al. investigated three cases where individuals without any evidence for novel ABCC6 mutation and containing no family history of PXE began to develop PXE like symptoms within few months after having liver transplant [10]. Investigations focused on carrying out various genomic studies on the patient’s own blood cells, kidney specimen and family members’ blood, liver and kidney specimen, which presented no evidence for containing ABCC6 mutation. Symptoms were only found to be developing after the liver transplant with only the donor liver cells expressing the novel ABCC6 mutations. Hence the conclusion was reached that unscreened liver transplants from PXE individuals resulted in the subjects to acquire PXE [10].
Individuals suffering from β-thalassemia or sickle cell anemia, especially of Mediterranean origin have been documented to exhibit PXE like symptoms [11]. β-thalassemia and sickle cell anemia are in-born haemoglobinopathies which contribute from mild to fatal anemic conditions in the suffering individuals. β-thalassemia inaugurates owing to mutations occurring in the β-globin gene that results in down regulation of β-hemoglobin chain production in red blood cells. To compensate for the underproduced β-hemoglobin chains, an increase in the α-hemoglobin chin production occurs, which disrupts the normal infrastructure and stability of the erythrocyte. This leads to transformation of erythrocytes into inclusion bodies (eventually leading to destruction of the erythrocytes) and ineffective erythropoiesis, which in turn leads to anemia [11]. Sickle cell anemia results from a single base nucleotide point mutation which results in the appointed amino acid which is glutamic acid to be altered to valine. This disrupts the erythrocyte surface charge and infrastructure in such a manner that the erythrocyte starts to exhibit a sickle shape, which deprives the oxygen capacity of the erythrocytes, leading to anemia [12]. Interestingly the PXE like symptoms develop independently of any novel mutations in the ABCC6 gene. Because of the haemoglobinopathies, the liver has a down regulation of ABCC6 protein resulting in ABCC6 protein deficiency [11]. The pathogenesis of the PXE like syndromes in β-thalassemia and sickle cell anemia individual is not known, however it is believed that a converging molecular/metabolic pathway alters the expression of ABCC6 or somehow disrupts the biologic properties of its by-product in the liver and/or kidney as a secondary consequence of the haemoglobinopathy [5,11].
Long term use of a medication, known as Penicillamine has a record of causing PXE like and Elastosis perforans serpiginosa (EPS) side-effects in patients [6]. Penicillamine is usually prescribed to patients with Wilson’s disease but can also be offered to patients suffering from, rheumatoid arthritis, cystinuria, primitive biliary cirrhosis and scleroderma. The drug acts as a copper chelating compound which happens to interfere with elastin maintenance and synthesis. The exact mechanism of how the drug interferes with the elastin metabolism is unclear and consideration to modify the drug to minimize or eliminate its side-effects remains uncertain [6]. Although several cases of acquiring PXE and EPS like symptoms due to long-term use of the drug have been reported, the literature is still very limited regarding the availability of data providing record of annual incidence of acquiring PXE through long term use of the drug and lack of follow up reports, investigations [13-15].
ABC transporter family
ABCC6 gene is a member of ATP-Binding Cassette (ABC) transporter family. ABC Transporter family proteins are ATPdependent pumps hence they are named ATP - binding cassette (ABC) transporter family. As well as being present in mammals, ABC transporter family proteins are also present in all kinds of prokaryotes in addition to plants, fungi i. e. yeast and other animals. In mammals, these proteins are predominantly involved in the liver as well as kidney, intestine, blood-brain barrier, blood - testis barrier and placenta [7]. The proteins are involved in transporting a range of endogenous substrates comprising sugars, amino acids, peptides, metal ions, inorganic ions and a multitude of hydrophobic compounds and metabolites facilitating their influx or efflux across membranes of cellular organelle vesicles as well as plasma membranes [7]. The significance of their involvement in cellular processes is highlighted by the fact that mutations occurring in atleast 11 sites of the ABC genes results in life-threatening inherited diseases, for example cystic fibrosis due to mutations in ABCC7 gene, certain types of Zellweger syndrome (ABCD3 and ABCD2), X - Linked adrenoleukodystrophy (ABCD1 & ABCD2) and PXE due to mutations occurring in ABCC6 gene [7].
There 49 designated genes of ABC in humans, which are divided into subfamilies of seven groups. The seven groups are assigned as A, B, C, D, E, F and G. Each member is assigned to perform specific and certain tasks within the cell to maintain normal cellular processes [7].
The ABC transporter protein falls under the category of ATPpowered pumps, hence its name ATP-Binding cassette (ABC) transporter protein. The ABCC6 protein is an ATP - powered pump which is among the many ATP - powered pumps constituting the ABC family. These pumps release energy by hydrolyzing ATP and utilize this energy to transfer biological molecules across the cell or organelle membrane against an electrochemical gradient [7].
ABC protein structure
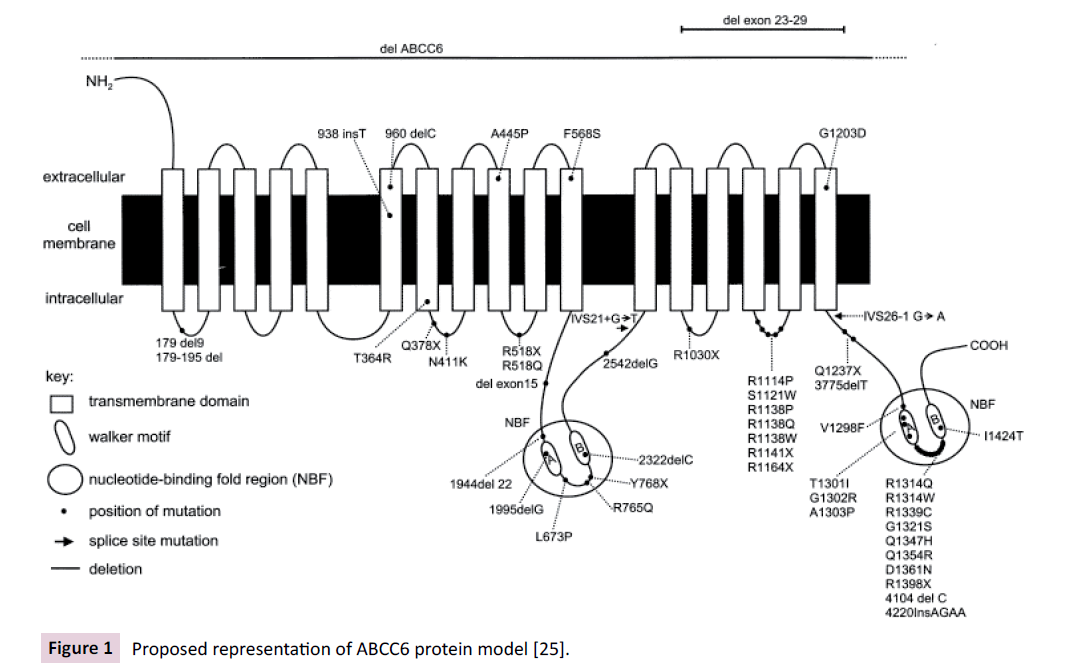
The quaternary infrastructure of the ABC transporter protein core is composed of total four domains, two nucleotide binding domains (NBDs also known as NBFs), and two Transmembrane Domains (TMDs) [7]. NBDs/NBFs have been described to contain 5 major highly conserved motifs, which are Walker A, Walker B, the ABC signature motif, the H loop and the Q loop. The two NBDs/NBFs bind together and perform hydrolyzation of ATP, indicating that the NBDs/NBFs account for some ATPase activity [16,17]. The two NBds/NBfs are contained within the ATP-Binding Cassette subfamily C number 6 protein (ABCC6) (Figure 1). The TDMs partake in substrate recognition and their translocation across the lipid membrane [7].
Subfamily member A: Member A is comprised of 12 genes, most which control lipid trafficking in many cell types and organs. ABCA proteins are found to one of the largest among ABC proteins and weigh more than 2100 amino acid in length. Mutations occurring in certain ABCA genes result in genetic diseases such as familial high-density lipoprotein (HDL) deficiency and Tangier disease T1.
Subfamily member B: Member B is comprised of 11 genes and is found to be unique to mammals. The 11 gene code for 4 full transporters and 7 half- transporters. More than a few of the B subfamily members are known to be associated with multi-drug resistance in cancer [18]. Mutations occurring in the ABCB genes has been recognized in contributing to X - linked sideroblastic anemia, diabetes type 2, ankylosing spondylitis, coeliac disease, several cholestatic liver diseases of infancy and lethal neonatal syndrome. Although connections have been found between the mentioned diseases and certain member B genes through genotype and phenotype associated studies, there is a possibility that the findings were false positives, hence overestimated. Therefore, investigations should be repeated among other large cohorts to confirm the association [7].
Subfamily member C: Member C is known for possessing the gene for cystic fibrosis. The gene is known with the gene connotations CFTR or ABCC7 [7]. Other members of the C family are known to cause Dubin-Johnson syndrome (ABCC2) and familial persistent hyperinsulinemic hypoglycemia of infancy (FPHHI) (ABCC8) [19,20]. ABCC6 have been found to be similar in terms of protein structure and sequence composition to ABCC1. ABCC1 is primarily involved in transmembrane transportation of polyanion-like substrates, which implies that ABCC6 might be involved in transmembrane transportation of polyanion-like substrates [21,22].
Genetic and protein structure of ABCC6
The gene of ABCC6 is composed of 13 exons, which spans about 73kb. The mRNA transcript has an open reading frame of 4.5kb, which is responsible for coding a protein sequence length of 1,503 amino acids. The molecular weight is theoretically believed to be 165 kD [21,22]. The synthesized protein is also recognized as a multi-drug resistance protein 6 (MRP6). The protein has been structurally found to have three hydrophobic membrane spanning domains, seventeen transmembrane helices, two conserved ATPBinding folds (NBFs). As mentioned earlier in the Introduction, the latter two regions of NBFs have been found to possess some ATPase activity [16,17]. Studies of mutational analysis of in several ABC protein regions have been shown to critically influence the infrastructure of the NBFs hence critically influences the activity of ATPase in ATP-driven transport functions (Figure 1) [16,23].
Role of ABCC6 and its association with excretory organs
ABCC6 transporter displays a variety of activities incorporating from cell signaling, ion channel and toxin (i.e., bacterial and fungal toxins) excreting activity, drug metabolism, protein secretion, antigen presentation as well as contributing towards antibiotic resistance [7]. The ABC transporter protein is also recognized as multi-drug resistance-associated protein 6 (MRP6) and has an ill repute for contributing towards to drug resistance in cancer patients [3,24]. Expression of this protein is very abundant in excretory organs such as the liver followed by the kidney, but surprisingly low in the affected tissues such as arterial blood vessel wall tissue and dermal fibroblasts [25]. The protein’s precise function in the liver and in other tissues is not known, however their very presence in these organs and in high abundance have led academics and researchers to consider that, they might be involved in cellular drug detoxification or functions as a conjugate glutathione pump or as a multi-specific organic anion transporter [26,27]. However, a study performed by Fulop et al. showed that ABCC6 protein does not transport vitamin K3- glutathione conjugate from the liver to circulation. Despite the abundant expression of the protein in liver and kidney, the organs are not affected as severely as the dermal tissues, eyes and vascular wall tissues by PXE [28]. This statement has a degree of inaccuracy since in some severe cases, ectopic mineralization in the liver has been documented as discussed earlier in the section Additional clinical manifestations of PXE [4,29].
Findings by Scheffer et al. have shown that the ABCC6 protein in the human liver is baso-laterally located in the membranes of hepatocytes. Their peculiar position in the hepatocytes and their distribution throughout the liver implies that ABCC6 is potentially pumping organic anions or alike substrates into the blood from the liver [30].
What are the possible theories for the pathophysiology of such ectopic mineralization?
PXE is also considered a metabolic disorder since the primary defect lies in the liver with the highest expression of ABCC6 protein in the liver [2,24,25].
Hu et al. discusses possible pathophysiological reasons behind PXE [2]. Due to the primary defect lying majorly in the liver and kidney, it is possible that ABCC6 substrates accumulate in abnormal levels which then modulates the elastic fiber assemblage at certain sites of the body. It is also possible that local ABCC6 tissue defects at certain numerous sites of the body result in typical PXE clinical manifestations. It is also possible that the typical clinical manifestation of PXE result due to a combination of both local and systemic ABCC6 defects.
What substrates do ABCC6 transfer?
It is not known what substrates are being transported by ABCC6 or substrates that temper with the normal metabolic pathways of mineralization [4]. However, academics and researchers have considered possibilities of potential substrates or membrane bound proteins such as matrix Gla protein (MGP) and calcium and phosphate [16,31].
A reduction in the vitamin K-dependent γ-glutamyl carboxylation of matrix Gla protein (MGP) was noticed. A fully carboxylated MGP is understood to act as a strong local anti-mineralization. Based on this observation, it was speculated that deficiency in Vitamin K or a derivative of Vitamin K could potentially be contributing to the ectopic mineralization, due to deficient γ-glutamyl carboxylation of MGP [16]. Thus, investigations were executed by various scientists [31,32]. Their findings proved the hypothesis wrong and moreover it was shown that ABCC6 does not transport vitamin K3-glutathione conjugate from the liver to circulation.
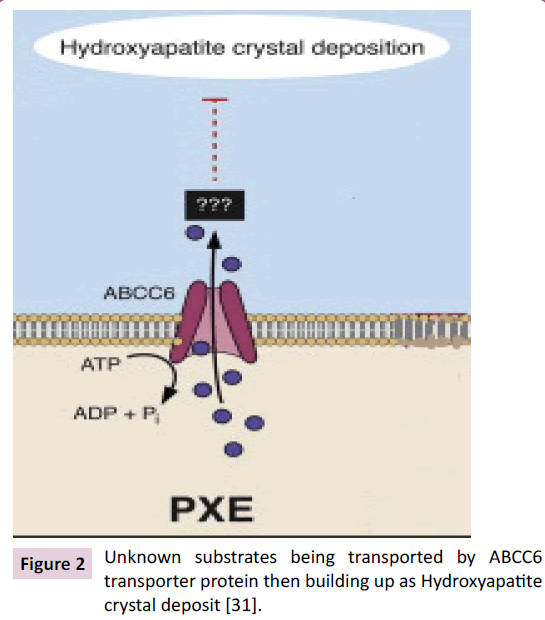
Histological examination of mineral deposits of lesions of PXE humans and ABCC6-/- mice displayed to contain hydroxyapatite, calcium and phosphate. It is also reported that the ratio of calcium/ phosphate significantly increases with maturing lesions. As the calcium and phosphate deposition builds up with the maturing lesion, the calcium and phosphate deposition transform into calcium phosphate to hydroxyapatite deposit. This suggests that ABCC6 is potentially involved in the metabolism or transportation of calcium and phosphate; however it was still not confirmed that these substrates are transported by ABCC6 and requires for more intensive studies to confirm it (Figure 2) [31].
Figure 2 Unknown substrates being transported by ABCC6 transporter protein then building up as Hydroxyapatite crystal deposit [31].
Subfamily member D: Member D or ABCD genes code for ALD or peroxisomal transporters present on mammalian peroxisomes. Peroxisomes drive the β-oxidation of very long chained fatty acids (VLCFA) and hence control lipid trafficking in cells and tissues [33]. ABCD genes are comprised of only 4 genes that code for halftransporters. Half-transporters are established from 3 subunits of heterodimers or homodimers. The 4 genes are understood to code for at least 49 discrete proteins. Mutations in the ABCD are recognized to cause certain form of Zellweger syndromes and X-linked adrenoleukodystrophy (X-ALD) [7].
Subfamily member E: Member E or ABCE1 is a unique member of the family as t is the only member which codes for an organic anion binding protein. The anion - binding protein does not function as a transporter protein due to absence of transmembrane domain, however does contain an ATP - binding protein [7]. Member E codes for 5 distinctive proteins through the process of 15 spliced transcripts. ABCE1 has also been found to interfere with ribonuclease L activity by blocking its activity. Activation of Ribonuclease L promotes antiviral combat against viral and other microbial infections [34]. Thus, ABCE1 appears to be involved in regulation of Ribonuclease L activity. Any mutation in this member of gene leading to an inherent disease is not reported [7].
Subfamily member F: Like member E, Member F is also known to possess ATP - Binding domains but no transmembrane membrane which makes member F unlikely to be involved in transportation activity. ABCF genes are known to code for 26 distinct proteins. The ABCF genes are assumed to be involved in playing a role in inflammatory responses since ABCF genes appear to be stimulated by tumor necrosis factor - α (TNF-α). Any mutation in this member of gene leading to an inherent disease is not reported [7].
Subfamily member G: ABCG genes comprised of G1, G4, G2, G5, G8, code for 18 distinct subunit proteins, which play a role in sterol maintenance. Mutations occurring in the ABCG proteins have been correlated with causing sterol accumulation disorders and atherosclerosis [7].
Discussion
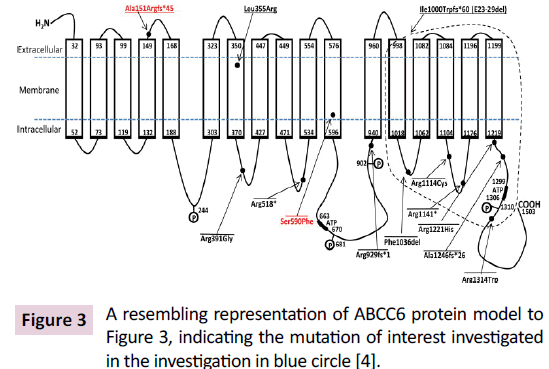
Interestingly, mutations in the ABCC6 gene that are known to contribute to PXE have also been found to contribute to another parallel but a distinct entity of disease in some patients (infants) called Generalized Arterial Calcification of Infancy (GACI). There are 13 distinct mutations in ABCC6 gene that are known to cause GACI, out of which 11 mutations have been found to overlap with PXE causing mutations [4]. Out of these 11 mutations, the mutation c. 1064T > G [p.Leu355Arg] is the subject of focus in this investigation. This mutation results in the replacement of Leucine with Arginine, which in turn causes alterations in folding of the ABC transmembrane protein structure due to change in the polarity of a conserved amino acid, Leucine to a positively charged amino acid, Arginine in the transmembrane domain (Figure 3) [4]. The gene is located at 16: 16202789 - 16201461 on the reverse strand. The mutation results in a T→G transition. Thus, an A→C transition would occur on the forward strand.
GACI is distinct disease from PXE characterized by early onset of disease process, whereas PXE is more known for its juvenile or late onset. GACI is also inherited in an autosomal recessive manner which lies in the category of rare genetic disorders, affecting 1 in 20000 newborn babies [35]. Characteristic manifestations are prenatal calcification of the internal elastic lamina along with ectopic mineralization of blood vessels and myointimal proliferation of muscular arteries contributing to arterial stenosis [4]. The disease is so progressive that majority of the infants with GACI do not survive beyond or die within the first year of life. This is mainly due to vascular complications [36]. Patients are also reported to suffer from severe physiological consequences such as hypertension. Sudden death can result from congestive cardiac failure and myocardial ischemia [4]. Very few patients survived the disease in neonatal period; however a recent invention of treating patients with bisphosphates has shown some satisfactory results [4]. GACI is typically caused due to mutations in the ENNP1 gene. However, a mutational analysis study of 14 patients who were affected by GACI showed that they had mutations in the ABCC6 gene but had no mutations that were identified in the ENNP1 gene [4].
Human Genome Project (HGP) is piloted by an international collaboration of scientists all around the world and can provide a great platform for investigating the etiology of various disease at the genetic level. HGP was approved and incited by the Department of Energy (DOE) in 1985, USA [37]. The National Institute of Health (NIH) and DOE together took the assignment to fund HGP which also involved 25 laboratories in 5 countries. The project officially commenced in 1990 for a time frame of 15 years with an estimated cost of US $ 200 million. Within this time frame, the objective of HGP was to determine the order of 3 billion DNA nucleotides through DNA sequencing. Both governments’ and private sectors’ arising social enthusiasm, strong support and curiosity to investigate the root cause of rare life-threatening conditions started to drive the progression of HGP. The ambition was achieved and completed in April 2003 [37].
Conclusion
HGP not only aids in discovering the genomic cause but also helps in early detection of rare inherited genetic disorders through newborn screening. It provides the opportunity to manufacture personalized medicines to suit specific treatment-related needs of individual patients [38]. Also, it assists in understanding genomic profile expression of certain patients as a response to certain medicines [39]. With emerging advancement in technology and genomic understanding, several gene-based therapy treatments such as Talens have been established and are implemented in patients with genetic conditions such as sickle cell anemia, Duchene muscular dystrophy [40-43]. Thus, HGP offers a great opportunity for investigating a rare genetic disorder such as Pseudoxanthoma elasticum (PXE).
References
- Terry SF (2013) Disease advocacy organizations catalyze translational research. Front Genet 4: 101.
- Uitto J, Váradi A, Bercovitch L, Terry PF, Terry SF (2013) Pseudoxanthoma elasticum: Progress in research toward treatment: summary of the 2012 PXE international research meeting. J Invest Dermatol 133: 1444-1449.
- Le Saux O, Urban Z, Tschuch C, Csiszar K, Bacchelli B (2000) Mutations in a gene encoding an ABC transporter cause Pseudoxanthoma elasticum. Nat Genet 25: 223-227.
- Nitschke Y, Baujat G, Botschen U, Wittkampf T, Du Moulin M (2012) Generalized arterial calcification of infancy and Pseudoxanthoma elasticum can be caused by mutations in either ENPP1 or ABCC6. Am J Hum Genet 90: 25-39.
- Fabbri E, Forni GL, Guerrini G, Borgna-Pignatti C (2009) Pseudoxanthoma-elasticum-like syndrome and thalassemia: an update. Dermatol Online J 15: 7.
- Na SY, Choi M, Kim MJ, Lee JH, Cho S (2010) Penicillamine-induced Elastosis Perforans Serpiginosa and Cutis Laxa in a Patient with Wilson's Disease. Ann Dermatol 22: 468-471.
- Vasiliou V, Vasiliou K, Nebert D (2009) Human ATP-binding cassette (ABC) transporter family. Hum Genomics 3: 281-290.
- Nebert DW, Zhang G, Vesell ES (2008) From human genetics and genomics to pharmacogenetics and pharmacogenomics: past lessons, future directions. Drug Metab Rev 40: 187-224.
- Pope F (1974) Autosomal dominant Pseudoxanthoma elasticum. J Med Genet 11: 152-157.
- Pope F (1974) Two types of autosomal recessive Pseudoxanthoma elasticum. Arch Dermatol 110: 209-212.
- Bercovitch L, Martin L, Chassaing N, Hefferon T, Bessis D, et al. (2011) Acquired Pseudoxanthoma elasticum presenting after liver transplantation. J Am Acad Dermatol 64: 873-878.
- Martin L, Douet V, VanWart CM, Heller MB, Le Saux O (2011) A Mouse Model of β-Thalassemia Shows a Liver-Specific Down-Regulation of Abcc6 Expression. Am J Pathol 178: 774-783.
- Hoffbrand AV, Moss PAH, Pettit JE (2006) Essential haematology. Blackwell Publications, Malden, Massachusetts, USA.
- Chassaing N, Martin L, Calvas P, Le Bert M, Hovnanian A (2005) Pseudoxanthoma elasticum: a clinical, pathophysiological and genetic update including 11 novel ABCC6 mutations. J Med Genet 42: 881-892.
- Poon E, Mason GH, Oh C (2008) Clinical and histological spectrum of elastotic changes induced by penicillamine. Australas J Dermatol 43: 147-150.
- Coatesworth AP, Darnton SJ, Green RM, Cayton RM, Antonakopoulos GN (1998) A case of systemic pseudo-Pseudoxanthoma elasticum with diverse symptomatology caused by long-term penicillamine use. J Clin Pathol 51: 169-171.
- Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: The multidrug resistance-associated proteins. J Natl Cancer Inst 92: 1295-1302.
- Higgins CF (1992) ABC transporters: From microorganisms to man. Annu Rev Cell Biol 8: 67-113.
- Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11: 1156-1166.
- Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, et al. (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271: 1126-1128.
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM (1995) Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science 268: 426-429.
- Belinsky MG, Kruh GD (1999) MOAT-E (ARA) is a full-length MRP/cMOAT subfamily transporter expressed in kidney and liver. Br J Cancer 80: 1342–1349.
- Kool M, Van der Linden M, De Haas M, Baas F, Borst P (1999) Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res 59: 175–182.
- Schneider E, Hunke S (1998) ATP-binding-cassette (ABC) transport systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains. FEMS Microbiol Rev 22: 1-20.
- Ringpfeil F, Pulkkinen L, Uitto J (2001) Molecular genetics of Pseudoxanthoma elasticum. Exp Dermatol 10: 221-228.
- Hu X, Plomp AS, Van Soest S, Wijnholds J, De Jong PT (2003) Pseudoxanthoma elasticum: A clinical, histopathological and molecular update. Surv Ophthalmol 48: 424-438.
- Jiang Q, Takahagi S, Uitto J (2012) Administration of bone marrow derived mesenchymal stem cells into the liver: Potential to rescue Pseudoxanthoma elasticum in a mouse model (Abcc6−/−). Biomed Res Int 12: 818937.
- Deeley RG, Cole SP (1997) Function, evolution and structure of multidrug resistance protein (MRP). Semin Cancer Biol 8: 193-204.
- Belinsky MG, Chen ZS, Shchaveleva I, Zeng H, Kruh GD (2002) Characterization of the drug resistance and transport properties of multidrug resistance protein 6 (MRP6, ABCC6). Cancer Res 62: 6172-6177.
- Korn S, Seilnacht J, Huth C, Feller AM (1987) Cardiovascular manifestation of Pseudoxanthoma elasticum (Gronblad-Strandberg syndrome). Thorac Cardiovasc Surg 35: 191-194.
- Scheffer GL, Hu X, Pijnenborg AC, Wijnholds J, Bergen AA, et al. (2002) MRP6 (ABCC6) detection in normal human tissues and tumors. Lab Invest 82: 515-518.
- Li Q, Uitto J (2013) Mineralization/Anti-mineralization networks in the skin and vascular connective tissues. Am J Pathol 183: 10-18.
- Le Saux O, Martin L, Aherrahrou Z, Leftheriotis G, Váradi A, et al. (2012) The molecular and physiological roles of ABCC6: more than meets the eye. Front Genet 3: 289.
- Morita M1, Imanaka T (2012) Peroxisomal ABC transporters: Structure, function and role in disease. Biochim Biophys Acta 1822: 1387-1396.
- Liang SL, Quirk D, Zhou A (2006) RNase L: Its biological roles and regulation. IUBMB Life 58: 508-514.
- Lorenz-Depiereux B, Schnabel D, Tiosano D, Häusler G, Strom TM (2010) Loss-of-function ENPP1 mutations cause both generalized arterial calcification of infancy and autosomal-recessive hypophosphatemic rickets. Am J Hum Genet 86: 267-272.
- Li Q, Jiang Q, Uitto J (2014) Ectopic mineralization disorders of the extracellular matrix of connective tissue: molecular genetics and pathomechanisms of aberrant calcification. Matrix Biol 33: 23-28.
- Human Genome Project (HGP) (2014) Available at: https://www.genome.gov/10001772.
- Hefti M, Beck A (2014) The human genome project and personalized medicine. Reference Module in Biomedical Sciences pp: 3418–3422.
- Marchionni L, Wilson RF, Marinopoulos SS, Wolff AC, Parmigiani G, et al. (2007) Impact of gene expression profiling tests on breast cancer outcomes. Evid Rep Technol Assess 160: 1-105.
- Ousterout D, Perez-Pinera P, Thakore P, Kabadi A, Brown M, et al. (2013) Reading frame correction by targeted genome editing restores dystrophin expression in cells from Duchene muscular dystrophy patients. Mol Ther 21: 1718-1726.
- Sun N, Liang J, Abil Z, Zhao H (2012) Optimized TAL effector nucleases (TALENs) for use in treatment of sickle cell disease. Mol Biosyst 8: 1255-1263.
- Nebert D, Zhang G Vesell E (2008) From human genetics and genomics to pharmacogenetics and pharmacogenomics: Past lessons, future directions. Drug Metab Rev 40: 187-224.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences