ISSN : 2348-9502
American Journal of Ethnomedicine
Histochemical Localization of Crystals and their Taxonomic Significance in Five Species of the Subfamily Papilioniodeae
Ojimba Chioma F*, Mbagwu Ferdinand N and Inyama Chiemeka N
Department of Plant Science and Biotechnology, Imo State University, Owerri, Nigeria
- *Corresponding Author:
- Ojimba Chioma F
Department of Plant Science and Biotechnology
Imo State University, Owerri, Nigeria
E-mail: chingor01@yahoo.com
Received Date: March 07, 2017; Accepted Date: March 28, 2017; Published Date: March 31, 2017
Citation: Chioma FO, Ferdinand NM, Chiemeka NI. Histochemical Localization of Crystals and their Taxonomic Significance in Five Species of the Subfamily Papilioniodeae. Am J Ethnomed. 2017, 4:1.
Abstract
An investigation into the histochemical localization of crystals and their taxonomic significance in five species of the subfamily Papilioniodeae was carried out. The result showed the presence of rhomboidal type of crystals in the leaf of M. aboensis and L. cyanescens; stellate type in C.pubescens but absent of crystals in C.retusa and I. spicata. The petiole of M. aboensis has stellate, rhomboidal and styloid types of crystals; I. spicata has crystal sand type; C. pubescens has both stellate and rhomboidal types; L. cyanescens has stellate but absence of all these types in C. retusa. At stem of these species, M. aboensis has stellate, rhomboidal and crystal sand crystals; I. spicata has rhomboidal; C. pubescens has stellate, rhomboidal and crystal sand crystals; L. cyanescens has only crystal sand type while C. retusa has none. There is absence of crystals at the root of these species. These results showed that M. aboensis and C. pubescens are closely related in terms of crystal types while C. retusa can be distinguished from the rest of the taxa investigated.
Keywords
Taxonomy; Papilionoideae; Identification; Histochemistry
Introduction
Leguminosae is usually divided into three subfamilies: Papilioniodeae, Caesalpinioideae and Mimosoideae because of its large size [1]. Milletta aboensis, Crotalaria Retusa, Indigofera spicata, Centrosema pubescens and Lonchocarpus cyanescens belong to the subfamily Papilionoideae. The subfamily is easily recognized by their characteristic Papilionanceous (butter fly-like) flower [2]. The Papilionoideae contains most of the important leguminous crop species such as soya bean (Glycine max), common pea (Pisum sativurn), chickpea (Cicer arietinim), French bean (Phaseolus vulgaris), Lentril (Lens culinaris) and peanut (Arachis hypogaea). Papilionoideae is the largest of the three subfamilies with about 476 genera and 13,855 species [3].
They are herbs, shrubs or trees. Leaves are pinnately or simple with inflorescence racemose and axillary. It is characterized by markedly bilaterally symmetrical, papilionoiaceous corolla. This means that 5 sepals and five petals are differentiated into one standard petal, two wing petals and a keel formed from two petals that are united along their ventral margins and enclosing the stamens and the pistil. Ovary is superior with marginal placentation. Fruit is a legume and seeds are non endospermic.
Floral formular of Papilionoideae
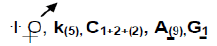
Some members of Leguminoseae (e.g. the beans) provide man and his livestock with a highly nutritional food resource. Species of many genera (eg Albizia, Afzelia, Daniella, Brechystegia, Afromosia, Berlinia) are very good for timber. Species of Copaifera and Lonchocarpus are sources of dyes. Species of some genera (eg Pterocarpus) are sources of leaf vegetables. Many members produce edible oil in large quantities eg. soyabean, groundnut. Many members of legumes yield important fibers, green manures and forages. Species of many genera (eg Brachystegra, Acacia etc.) are used to produce commercial gum. Some seed species (.eg Brachystegia earycoma, Mucuna aurens are used for thickening soup. Many members of the family are grown as ornamentals eg. Crotalaria and Dilonixca esalpinia. All the tree species are important sources of firewood.
The botanical literature shows that the use of histochemistry in taxonomic conclusions is now a common practice. For example, the occurrence of calcium oxalates crystals in various plant families have been reported by various authors. Edeoga and Okoli [4], Ayensu [5], Okoli and Green [6], observed the presence of calcium oxalate in the chloroplasts and trichomes of some Dioscorea species and suggested that they could be employed for taxonomic purposes. Tomlinson [7], Edeoga and Ogbebor [8] equally reported that calcium oxalate crystals characterize different members of Commelina species and Zingeberales and reported their diverse distribution in most parts of the plants. The deployment of histochemistry as a tool for identification and classification of plants has been indicated in various families such as Verbenaceae Mathew and Shah [9], Dioscoreaceae Okoli and Green [10], Edeoga and Okoli, [4] Cucurbitaceae Okoli [6] and Commelinaceae [11]. Other authors that have used ergastic substances for clarity in taxonomy at all hierarchies are Itoh and Karg [12], Burkill [13], Wu and Huang [14] in Moraceae.. Crystals of calcium oxalate which occur in organs and tissues of many plant species are among the most widespread ergastic substance known in Angiosperm [15].
Calcuim oxalate crystals exist in different forms such as raphides, bundle of needles, styloids, elongated columnar crystals, druses, spheroidal, aggregates of prismatic crystals and crystals sand [16]. Itoh and Kang [12] found calcium oxalate crystal in the intercellular or middle lamella of the radial walls of the secondary phloem in the Taxodiaceae family. Calcuim oxalate crystal occurring in vertical parenchymatous cells include, the raphids of Morinda citrifolia and Hamella patterns, the solitary crystals of Psidium guajava and Anogeissus acuminate, the crystal sand of Cordia subcordata and Santivia tomentosa.
The taxonomic importance of calcium oxalate crystals found in leaves, stems and roots of most taxa lies in the variation in their shape and localization. For example, the rhomboidal crystal of Aneilema acquinociale and A. beniniense were different from the irregularly shaped oxalate crystals of A. paludosum and A. umbrosum. From these findings, these taxa could be separated on the basis of crystal shape. The localization of crystals within the root cortex is also important as this region in addition to its storage functions offers protection against desiccation. The persistent association of the crystals with the ground tissues (supportive), cortex (storage) and mesophyll (nutritional) clearly attests to the possible roles of calcium oxalate in the taxonomy and classification of Aneilema species.
The use of histochemical attribute of plant in taxonomic conclusions are recently becoming frequently. Edeoga and Ugbo [11], Edeoga and Okoli [4] had suggested that these ergastic substances could have nutritional, mechanical and transport roles in plants.
It is very unfortunate that despite the contributions of the above authors on crystals, little or no information exist on the shape of crystals that are found on the subfamily Papilioniodeae hence the need for this work with the objective of finding the type and shape of crystals present in the subfamily. Thus using the crystal type to characterize and identify the species.
Materials and Methods
Specimen collection
The five species used in the investigation namely Millettia aboensis, Crotalaria retusa, Indigofera spicata, Centrosema pubescens, and Lonchocarpus cyanescaes were all collected from Imo State. Millettia aboensis and Crotalaria retusa were collected from Okigwe Local Government Area while Indigofera spicata, Centrosema pubescens, and Lonchocarpus cyanescens were collected from Agriculture farm of Imo State University.
Specimen identification
The specimens were identified by a taxonomist, Professor S. E. Okeke and were confirmed at Forest Herbarium institute (FHI) Ibadan, Oyo State. Voucher specimens were deposited at Imo State University Herberium (IMSUH) Owerri. With Voucher numbers IMSUH 89,90,91,92 and 93 respectively.
Anatomical studies
Mature and fresh parts of the leaves, petioles and the stems were collected and sectioned. The cut sections of the fresh, leaves, petioles and stems were done at various portions. For the leaves, cutting was done from the middle portion, the petioles, cutting was at 0.5 cm from the node; while that of stem was 4 cm from the terminal portion. Before sectioning commences, the specimens undergo a pretreatment process referred to as killing and fixing. The aim of this process was to terminate suddenly and permanently, all life processes within the specimens and preserve the cells composing the materials as close to their original conditions as possible [17]. For the pretreatment, the specimens were treated using Formalin Acetic Alcohol (FAA) for 48 hours and then washed thoroughly in distilled water. The specimens were washed in two changes of 30% ethanol and are dehydrated in a graded series of ethanol (30%-50%-70%-95%) for at least 15 minutes in each grade.
They were covered for 3 hours in each of the following solutions containing a ratio of absolute alcohol to pure chloroform (v/v: 3:1, 1:1, 1:3), then pure chloroform. At the stage of pure chloroform, wax pellets (60°C melting point) were added and the wax is changed periodically. The containers of the specimen were transferred to an oven for 2-7 days to remove the chloroform. The contents of the vial were carefully transferred into moulds. The specimens were arranged using a flamed (hot) mounting needle and transferred to a cold water bath where it remains until the wax is sufficiently solid and was later stored in a refrigerator for two days.
A very thin section of 10-20 μ was made using a Reichert rotary microtome. Ribbon cut sections obtained from the microtome sectioning were placed on clean slides covered with a thin film of Haupts albumen and allowed to dry. Three or four drops of distilled water were added before mounting. The slides were placed on a hot plate at 40°C for few minutes kept overnight.
The slides were immersed in pure xylene for 2-5 minutes in a solution of xylene and absolute alcohol with a ration of 1:1 (v/v). They were later transferred to another solution of xylene and alcohol graded series (95%, 90%, 70% and 50%) in the ratio of 3:3 (v/v) for few minutes. Drops of safranin were used to stain the section for 5 minutes, washed off with distilled water and then counter stained with alcian blue for 2 minutes and then dehydrate in 50% alcohol, 70%, 80% and 90% alcohol solution respectively and pure xylene at intervals.
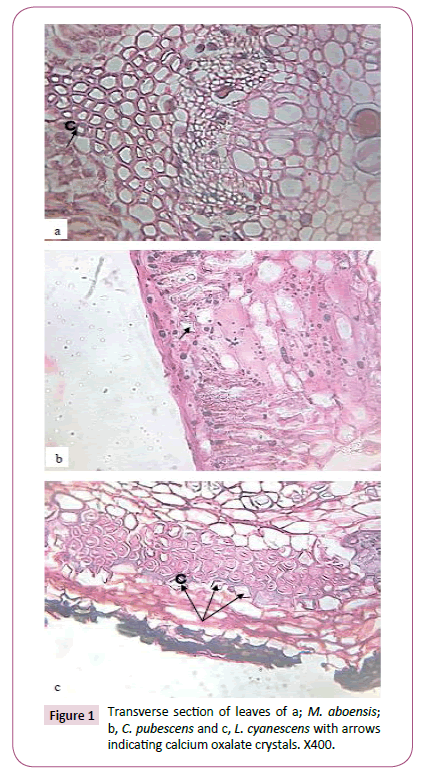
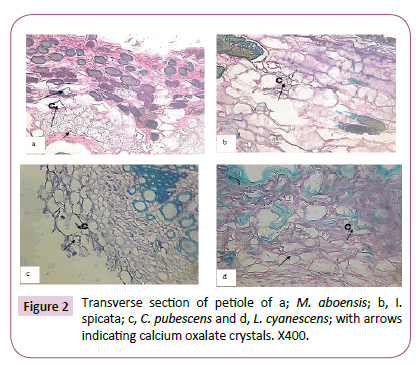
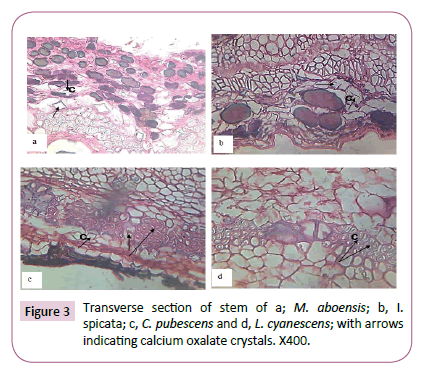
Drop of D.P.X mountant were introduced on to the slides enough to cover the length of the sectioned material and then covered with cover slip. The slides containing the mounted sections were placed on a hot plate at 30°C to dry. Photographs of materials sectioned were taken using a Leitz wetzler ortholux digital microscope filled with a camera (Figures 1-3).
Results
The presence of calcium oxalate crystals in some parts of the five taxa investigated was observed and recorded in Table 1. In the mesophyll of the leaves of M. aboensis and L. cyanescens, rhomboidal shaped crystals was observed and stellate were observed in C. pubescens while no crystal was observed in the other two taxa (Figure 1). At the petiole stellate, rhomboid and styloid shaped crystals was observed for M. aboensis, crystal sand crystal was found in I. spicata, in C. pubescens, stellate and rhomboid shaped crystals were observed, in L. cyanescens, it was stellate shaped crystal while no crystal was observed in C. retusa (Figure 2). In the stems, M. aboensis and C .pubescens have three different shapes of crystals: stellate shaped, rhomboid and crystal sand shapes, in I. spicata, shaped of crystal was rhomboid, L. cyanescens has only crystal sand shapes while no crystal was observed in C. retusa (Figure 3).
Table 1: Histochemical studies of the five species of the subfamily Papilionoideae investigated.
| Part of Plant | M.aboensis | C.retusa | I.spicata | C.pubescens | L.cyanescens |
|---|---|---|---|---|---|
| Leaf | Rhomboidal | - | - | Stellate | Rhomboidal |
| Petiole | Stellate, | - | Crystalsand | Stellate, | Stellate, |
| Rhomboidal, | Rhomboidal, | ||||
| Styloid | |||||
| Stem | Stellate | - | Rhomboidal | Stellate | Crystalsand |
| Rhomboidal, | Rhomboidal, | ||||
| Crystalsand | Crystalsand |
Discussion
The variation in shape of calcium oxalate crystals is of taxonomic importance and could be used to distinguish and classify different plant species. The leaf anatomy showed that M. aboensis and L. cyanescens have rhomboidal crystal; for C. pubescens has stellate crystal, while no crystal was observed on the other two taxa. This is of systematic importance as it delineates the species. At the petiole, M. aboensis, C. pubescens and L. cyanescens have star-like crystals. In addition, M. aboensis and C. pubescens have rhomboidal crystals; M. aboensis could be separated from the other taxa due to the presence of styloid observed. Also, crystal sand can be used to delimitate I. spicata from the other taxa while the absence of crystals at C. retusa further separates it from the other taxa investigated.
Only rhomboidal shaped crystals were observed in I. spicata in the stem anatomy is considered. Also, the presence of crystal sand separates L. cyanescens from the other taxa. M. aboensis and C. pubescens showed close affinity by having star-like rhomboidal and crystal sand crystals in common while C. retusa was delineated due to absence of calcium oxalate crystal.. The absence of crystals at the leaf, petiole and stem of C. retusa is a distinctive character of this taxon and thus separates it from the other four taxa [18]. The presence of calcium oxalate in four of the taxa investigated showed close phylogenetic relationship between them. While different shapes of the crystal observed could explain why they are placed under different genera orspecies. Authors such as Edeoga and Okoli [4] used shapes of crystals to distinguish species of Dioscorea; Okoli and McEuen also distinguished species of Cucurbitaceae using crystal shapes.
Conclusion
The data accrued from histochemical study of these species investigated however presented some important characters that could be exploited in improving the characterization of the five species. It was observed that calcium oxalate crystals are present in one form or the other among the taxa considered. Lack of crystals in the vegetative organ distinguished C. retusa from the rest of the taxa investigated. I. spicata can be separated from others because of the absence of crystal in its leaves. Therefore, this study has strengthened intra specie relationships among the species investigated and the usefulness of histochemistry in delimitation and identification of taxa.
References
- Heywood VH, Davis PH (1973) Principles of Angiosperm Taxonomy. Oliver and Boyd, London.
- Dutta AC (2005) Botany for Degree Students. Leth Edition, Oxford University Press.
- Hutchinson J, Dalziel JM (1954) Flora of West Tropical Africa. Vol. I and II. Crown Agenda for Overseas Government and Administration, London.
- Edeoga HO, Okoli BE (1995) Histochemical Studies in the Leaves of some Diascorea, L (Dioscoreaceae) and the taxonomic importance. Feddes Report 106: 113-120.
- Ayensu ES (1972) Anatomy of the Monocotyledons. VI. Dioscoreales. Oxford Univ. Press, New York.
- Okoli BE (1987) Anatomical Studies of the Leaf and Probract of Telfaira. Hooker (Cucurbitaceae). Feddes Report. 98: 231-239.
- Tomlinson P (1969) Anatomy of Monocotyledons, Commelinales Zingiberales. Clarendon Press. Oxford.
- Edeoga HO, Ogbebor NO (1999) Vegetative Anatomy in some Nigeria Species of Aneilema (Commelinaceae). Acta phytotax Geobot 50: 51-58.
- Matthew L, Shah GL (1984) Crystals and their Taxonomic Significance in some Verbebaceae. 83: 279-289.
- Okoli BE, Green BO (1987) Histochemical Localization of Calcium Oxalate Crystals in Starch Grains of Yams. (Dioscorea L) 60: 391-394.
- Edeoga HO, Ugbo HM (1997) Histochemical Localization of Calcium Oxalate Crystal in the Leaf Epidermis of some Commelina, L (Commelinaceae) and its bearing on Taxonomy. Acta Phytotax. Geobot, 48: 23-30.
- Itoh T, Kang KD (1993) The occurrence of Calcium Oxalate Crystals in the Cell Walls of the Secondary Phloem of Taxdiaceae. Holzforchung 47: 465-472.
- Burkill HM (1995) The useful Plants on West Tropical Africa. In: Families JL (ed.) Royal Botanic Gardens (2ndedn) Vol. 3, Kew, Richimond, UK.
- Wu-Chichih C, Kuo Hang LL (1997) Calcium Crystals in the Leaves of some Species of Moraceae. Bot Bull. Aca. Sin. 38: 97-104.
- Metcalf CR, Chalk L (1950) Anatomy of the Dicotyledons. Vol 1 and II. Crown agent for Overseas Government and Administration. London.
- Al-Rais AH, Meyers A, Watson L (1971) The Isolation and Properties of Oxalate Crystals from Plants. Ann. Bot 35: 1213-1218.
- Peacork EA (1973) Elementary Microtechique. London: Edward Arnoid.
- Gill LS (1988) Taxonomy of Flowering Plants. Onitsha. Africana Feb. Publishers.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences