ISSN : 2575-7725
Journal of Stem Cell Biology and Transplantation
Effect of Allogenic Bone Marrow- Versus Adipose Tissue Derived Mesenchymal Stem Cells in Treatment of Experimental Skeletal Muscle Injury in Adult Female Albino Rats: A Comparative Study
Department of Histology and Cell Biology, Faculty of Medicine, Ain Shams University, Egypt
- *Corresponding Author:
- Ghada Galal Hamam
Department of Histology and Cell Biology
Faculty of Medicine, Ain Shams University,Egypt.
Tel: 90020 01003960601
E-mail: ghada.hamam@yahoo.com
Received date: April 08, 2019; Accepted date: April 23, 2019; Published date: May 02, 2019
Citation: Abd Elaziz A, Moussa MH, Hamam GG, El-Waseef DAA (2019) Effect of Allogenic Bone Marrow- Versus Adipose Tissue Derived Mesenchymal Stem Cells in Treatment of Experimental Skeletal Muscle Injury in Adult Female Albino Rats: A Comparative Study. J Stem Cell Biol Transplant Vol.3 No.1:3 doi: 10.21767/2575-7725.100024
Copyright: © 2019 Abd Elaziz A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Skeletal muscle injury results from a variety of mechanisms as contusion, strain, laceration, or a combination of more than one mechanism. Skeletal muscle trauma is associated with development of fibrous tissue scar. Stem cells transplantation could improve skeletal muscle regeneration.
Aim of the work: to compare the effect of bone marrow versus adipose tissue derived mesenchymal stem cells in treatment of skeletal muscle injury in female albino rats.
Materials and Methods: Forty adult female albino rats weighing 150-200 gm were divided into three groups: Group I (negative control), Group II where the experiment lasted for ten days and group III where the experiment lasted for 8 weeks. Rats of group II and III were subjected to skeletal muscle laceration injury then were divided into three subgroups: subgroup a: rats were left for spontaneous healing, subgroup b: rats received a single intramuscular injection of 106 Bone marrow mesenchymal stem cells (BM-MSCs) at the site of injury, subgroup c: rats received a single intramuscular injection of 106 Adipose tissue derived stem cells (ADSCs) at site of injury. Skeletal muscle biopsies were prepared for histological examination using H&E stain.
Results: untreated skeletal muscle laceration group showed a gap filled with granulation tissue and mononuclear cellular infiltration with deposition of collagen fibers (fibrosis). The BM-MSCs treated groups showed regeneration of muscle fibers with decrease collagen fibers. Meanwhile, the ADSCs treated group showed better results regarding the arrangement of the regenerated muscle fibers.
Conclusion: Intramuscular injection of ADSCs is preferable than BM-MSCs in the treatment of skeletal muscle laceration injury.
Keywords:
Skeletal muscle; Laceration; BM-MSCs; ADSCs; Rat
Introduction
Human skeletal muscle constitutes about 40% of the body mass. Satellite cells (SCs) are skeletal muscle stem cells located between the plasma membrane of myofibers and the basal lamina. Their regenerative capacities are essential to repair skeletal muscle after injury [1,2]. Satellite cells represent around 5-10% of skeletal muscle cells depending on species, age, muscle location, and muscle type. In adult muscles, SCs are found in a quiescent state [3].
Skeletal muscle injuries can result from a variety of events, including direct trauma as muscle lacerations and contusions, indirect insult as strains and also can result from degenerative diseases as muscular dystrophies [4-6]. Skeletal muscle can regenerate completely and spontaneously in response to minor injuries, as strain. In contrast, after severe injuries, muscle healing is incomplete, resulting in the formation of fibrotic tissue that impairs muscle function. Although researchers have extensively investigated various approaches to improve muscle healing, there is still no gold standard treatment [7].
Over the last couple of decades, the field of regenerative medicine emerged as a novel promising strategy to treat many diseases. Mesenchymal stem cells (MSCs) are present in many adult tissues and can be easily isolated from different sources, including the bone marrow, adipose tissue, and umbilical cord, becoming an ideal candidate for cell-based therapy. Adipose tissue and bone marrow are the most available sources of MSCs, and both show the same immunoregulatory properties [8,9].
Aim of the Work
The present study was designed to compare between the effect of both bone marrow and adipose tissue derived mesenchymal stem cells on the healing of experimental skeletal muscle laceration injury in adult female albino rats.
Methods
Animals
This study was conducted on 40 adult female albino Wister rats weighing 150-200 g. Their ages ranged from two to three months. Five young male rats weighing 50-70 g were used as a source of allogenic bone marrow and adipose tissue, for stem cells separation. Animals were housed in clean plastic cages with mesh wire covers and were given a free access to standard rat chow diet and tap water for the period of experiment. They were kept under proper conditions of light, temperature and humidity. The experiment took place in the Medical Research Center, Faculty of Medicine, Ain Shams University. All the experimental procedures were performed in accordance with international standards for animal care. The period of the study was from June 2017 till March 2019.
Experimental protocol
After seven days acclimatization period, female rats were randomly divided into the following groups:
Group I (negative control): Included 10 rats. They were anesthetized, skin incision was done over the right gluteal muscle, and then they received local intra-muscular (IM) injection of 1 ml phosphate buffer saline (PBS). This group was subdivided into two subgroups, five animals each.
Subgroup Ia: muscle biopsies were taken ten days after the beginning of the experiment.
Subgroup Ib: muscle biopsies were taken eight weeks after the beginning of the experiment.
Group II (The experiment lasted for ten days): Included 15 rats that were subjected to right gluteal muscle laceration injury. Then rats were further subdivided into three subgroups five animals each.
Subgroup IIa (laceration): rats concomitantly received local intramuscular injection of 1 ml PBS at site of injury. Suturing was done, and then they were left for spontaneous healing.
Subgroup IIb (BM-MSCs treated): Rats concomitantly received local intramuscular injection of 1 ml PBS containing 1 × 106 BMMSCs at the site of injury before suturing.
Subgroup IIc (ADSCs): Rats concomitantly received local intramuscular injection of 1 ml PBS containing 1×106 ADSCs at the site of injury before suturing.
Muscle biopsies were taken from all rats ten days after the surgery and injection.
Group III (The experiment lasted for eight weeks): Included 15 rats that were subjected to right gluteal muscle laceration injury. This group was further subdivided into three subgroups five animals each:
Subgroup IIIa (laceration): rats concomitantly received local intramuscular injection of 1 ml PBS at site of injury. Suturing was done, and then they were left for spontaneous healing.
Subgroup IIIb (BM-MSCs): Rats concomitantly received local intramuscular injection of 1 ml PBS containing 1 × 106 BM-MSCs at the site of injury before suturing.
Subgroup IIIc (ADSCs): Rats concomitantly received local intramuscular injection of 1 ml PBS containing 1×106 ADSCs at the site of injury before suturing.
Muscle biopsies were taken from all rats eight weeks following surgery and injection.
Procedure of skeletal muscle laceration
Rats were anaesthetized by intra-peritoneal injection of a mixture of ketamine (50 mg/Kg) and xylazine (5 mg/Kg). Skin over the right gluteal muscle was shaved and sterilized. A laceration injury was done in the middle of the right gluteal muscle at about 0.5 cm depth using scissors. After the procedure a suture was taken between the ends of the lacerated gluteal muscle then the skin was sutured. This procedure was done according to mechanical laceration injury procedure performed by Pecanha but in gluteal muscle [10].
Sample collection
At the end of the experiment, animals were sacrificed by decapitation after ether inhalation anesthesia. Right gluteal muscle was taken from all rats including the injured area in the middle.
Preparation of tissue
Muscle specimens were fixed in 10% buffered formalin [11]. Fixation of specimens was done in situ to avoid muscle contraction. This was followed by dehydration, clearing and embedding in paraffin. Serial sections of 5 μm thickness were cut and stained with Hematoxylin and eosin (H&E).
Culture and isolation of stem cells
Isolation, culturing and characterization of BM-MSCs and ADSCs were done [12,13]. All steps were carried out at Stem cell research unit, Histology and Cell Biology department, Faculty of Medicine, Ain Shams University. The concentration of the obtained MSCs was 1 X 106 in 0.5 ml PBS.
Characterization of stem cells
Expression of the MSCs marker CD146 (in BM-MSCs) and CD34 (in ADSCs) were quantified by flow cytometry.
Morphometric and statistical study
The mean area parentage of collagen fibers was measured in Mallory`s trichrome stained sections from all groups. Measurements were taken from five different slides obtained from each animal. Five haphazard non-overlapping fields were examined for each slide. Five different readings from were counted and the mean was calculated for each specimen. An image analyzer Leica Q win V.3 program installed on a computer in Histology and Cell Biology Department, Faculty of Medicine, Ain Shams University was used. The computer was connected to a Leica DM2500 microscope (Wetzlar, Germany).
Data were collected and subjected to statistical analysis using one-way analysis of variance (ANOVA) performed with SPSS.21 program (IBM Inc., Chicago, Illinois, USA). Summary of the data was expressed as mean and standard deviation (SD). The significance of the data was determined by the P value. P values greater than 0.05 were considered non-significant, and P values less than 0.05 were considered significant.
Results
A) Morphology of primary culture of BM-MSCs and ADSCs
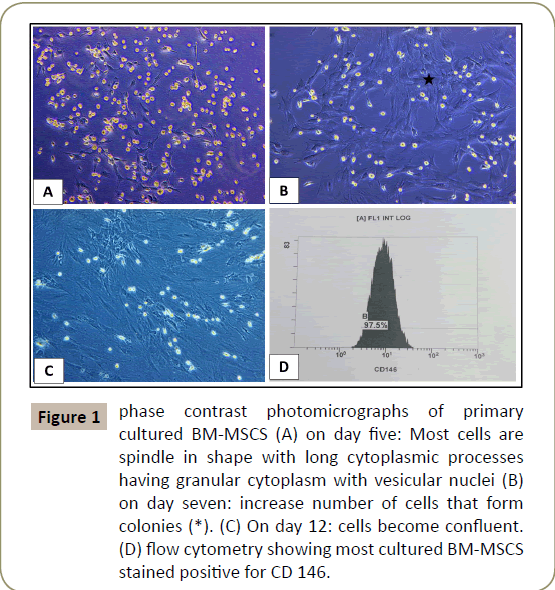
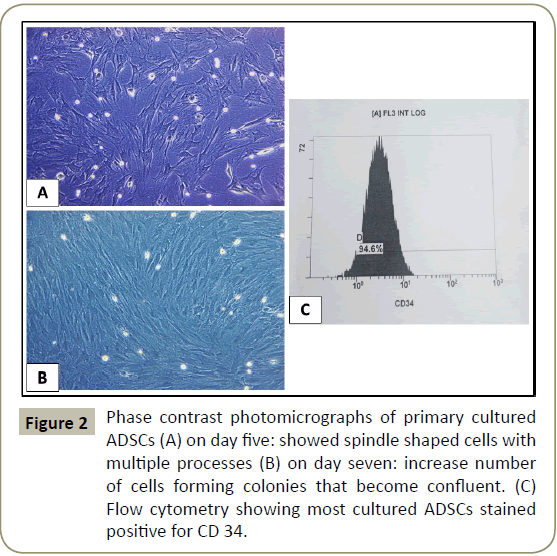
Was done using inverted phase contrast microscope. Examination of the primary cultures of BM-MSCs and ADSCs, BM-MSCs on day five, showed spindle shaped attached cells with multiple processes that increased in number and formed colonies, on day seven and became confluent on day 12, (Figures 1 and 2). ADCSs showed higher rate reaching confluence within a week.
Figure 1: phase contrast photomicrographs of primary cultured BM-MSCS (A) on day five: Most cells are spindle in shape with long cytoplasmic processes having granular cytoplasm with vesicular nuclei (B) on day seven: increase number of cells that form colonies (*). (C) On day 12: cells become confluent. (D) flow cytometry showing most cultured BM-MSCS stained positive for CD 146.
B) Characterization of stem cells
Characterizing the cultured cells after the 3rd passage using flow cytometry for BM-MSCs and ADSCs revealed that most of the cultured cells stained positive for CD 146 and CD 34 (Figures 1D and 2C respectively).
C) Histological results
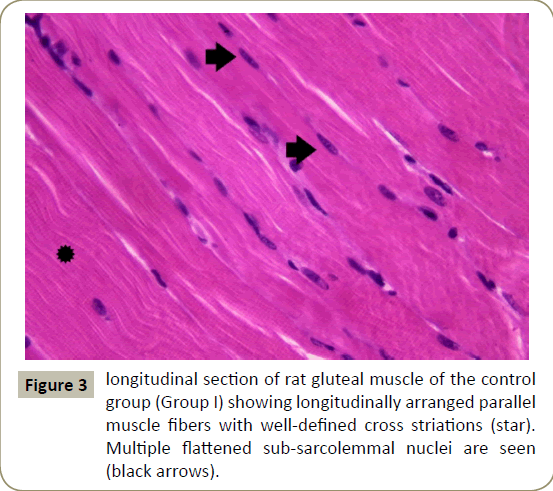
Examination of longuitdinal section of right gluteal muscle of control group (subgroup Ia and Ib) showed similar results. Longitudinal muscle fibers were seen with acidophilic cytoplasm multiple flattened peripheral nuclei and well-defined cross striations (Figure 3).
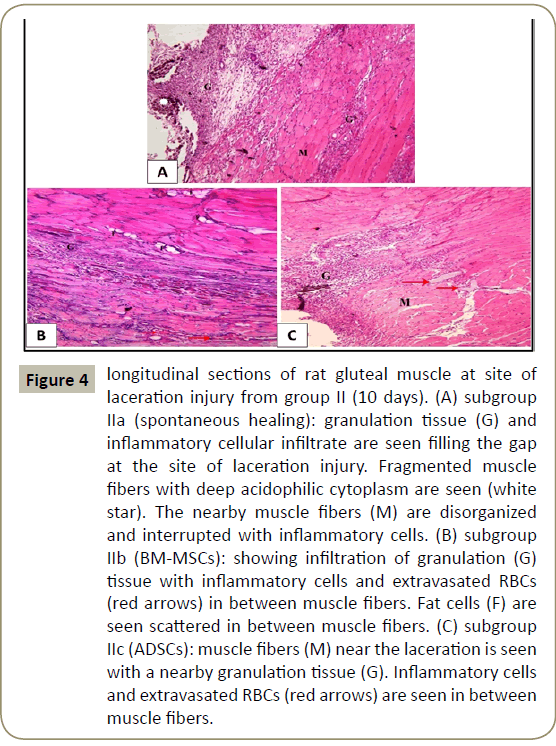
In group II that lasted for ten days, examination of H&E stained sections of subgroup IIa (spontanpus healing) showed granulation tissue and inflammatory cellular infiltrate filling the gap at the site of laceration injury. Fragmented muscle fibers were seen with deep acidophilic cytoplasm. The nearby muscle fibers appeared disorganized and interrupted with inflammatory cells (Figure 4A). In subgroup IIb (BM-MSCs), less granulation tissue, fewer inflammatory cells and extravasated red blood cells (RBCs) were seen. Nearby skeletal muscle fibers were seen with fat cells in-between (Figure 4B). While in subgroup IIc (ADSCs), fewer inflammatory cells and extravasted RBCs were seen in the granulation tissue of the defect (Figure 4C).
Figure 4: longitudinal sections of rat gluteal muscle at site of laceration injury from group II (10 days). (A) subgroup IIa (spontaneous healing): granulation tissue (G) and inflammatory cellular infiltrate are seen filling the gap at the site of laceration injury. Fragmented muscle fibers with deep acidophilic cytoplasm are seen (white star). The nearby muscle fibers (M) are disorganized and interrupted with inflammatory cells. (B) subgroup IIb (BM-MSCs): showing infiltration of granulation (G) tissue with inflammatory cells and extravasated RBCs (red arrows) in between muscle fibers. Fat cells (F) are seen scattered in between muscle fibers. (C) subgroup IIc (ADSCs): muscle fibers (M) near the laceration is seen with a nearby granulation tissue (G). Inflammatory cells and extravasated RBCs (red arrows) are seen in between muscle fibers.
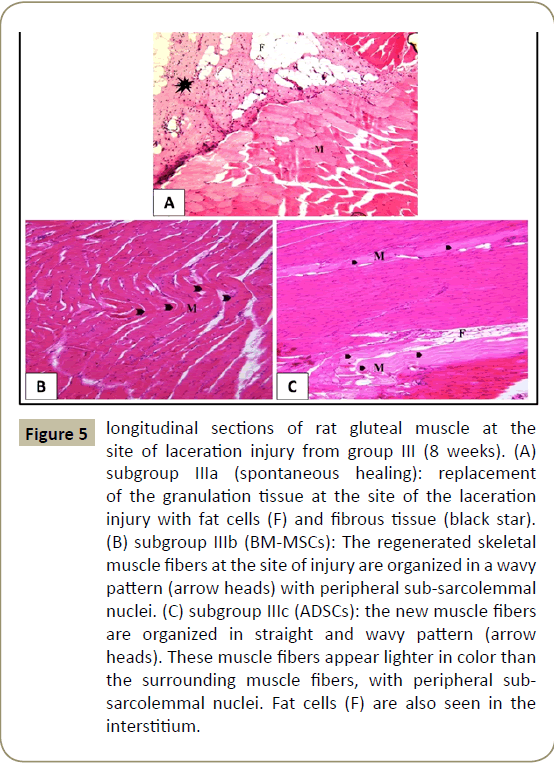
In group III that lasted for eight weeks, examination of H&E stained sections of subgroup IIIa (spontanpus healing) showed replacement of the granulation tissue at the site of the laceration injury with fat cells and fibrous tissue (Figure 5A). In subgroup IIIb (BM-MSCs), regeneration of skeletal muscle at the site of laceration injury appeared with newly formed muscle fibers that were organized in a wavy pattern with peripheral flattened nuclei (Figure 5B). While in subgroup IIIc (ADSCs), the new muscle fibers were organized in straight pattern, few fibers were seen wavy. The newly formed muscle fibers appeared lighter in color than the surrounding muscle fibers, with peripheral flattened nuclei. Fat cells were also seen in the interstitium (Figure 5C).
Figure 5: longitudinal sections of rat gluteal muscle at the site of laceration injury from group III (8 weeks). (A) subgroup IIIa (spontaneous healing): replacement of the granulation tissue at the site of the laceration injury with fat cells (F) and fibrous tissue (black star). (B) subgroup IIIb (BM-MSCs): The regenerated skeletal muscle fibers at the site of injury are organized in a wavy pattern (arrow heads) with peripheral sub-sarcolemmal nuclei. (C) subgroup IIIc (ADSCs): the new muscle fibers are organized in straight and wavy pattern (arrow heads). These muscle fibers appear lighter in color than the surrounding muscle fibers, with peripheral subsarcolemmal nuclei. Fat cells (F) are also seen in the interstitium.
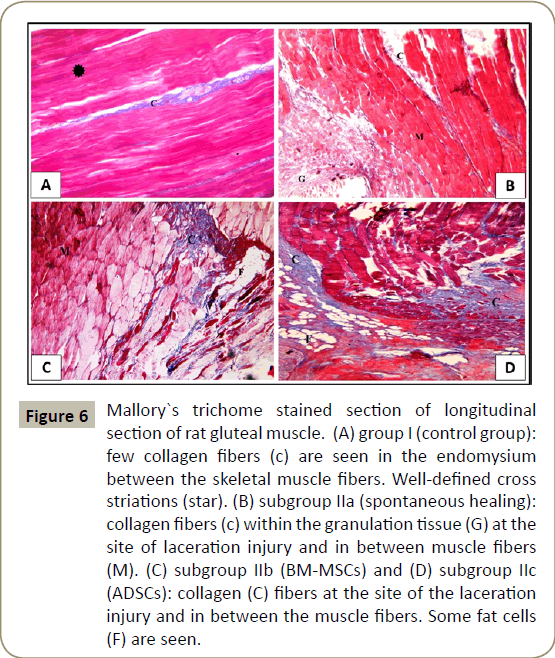
Examination of Mallory`s trichrome stained sections of control group (group I), showed few collagen fibers in the perimysium between the skeletal muscle fibers (Figure 6A). In subgroup IIa (spontaneous healing 10 days), collagen fibers were seen in the endomysium between muscle fibers and in the granulation tissue at the site of laceration injury (Figure 6B). In subgroup IIb (BM-MSCs for 10 days) and subgroup IIc (ADSCs for 10 days), apparently increased amounts of collagen fibers were seen at the site of the laceration injury (Figure 6C and D) respectively).
Figure 6: Mallory`s trichome stained section of longitudinal section of rat gluteal muscle. (A) group I (control group): few collagen fibers (c) are seen in the endomysium between the skeletal muscle fibers. Well-defined cross
striations (star). (B) subgroup IIa (spontaneous healing): collagen fibers (c) within the granulation tissue (G) at the site of laceration injury and in between muscle fibers (M). (C) subgroup IIb (BM-MSCs) and (D) subgroup IIc
(ADSCs): collagen (C) fibers at the site of the laceration injury and in between the muscle fibers. Some fat cells (F) are seen.
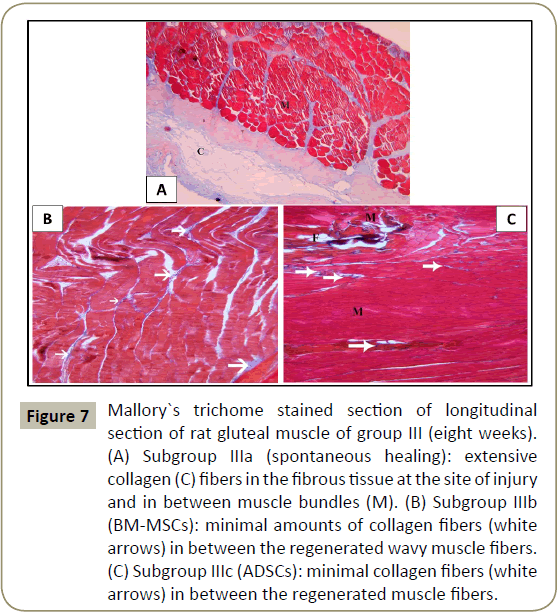
In group III that lasted for eight weeks, Mallory`s trichrome stained sections of subgroup IIIa (spontaneous healing), showed extensive collagen fibers at the site of injury (Figure 7A). In subgroups IIIb (BM-MSCs) and IIIc (ADSCs), minimal amounts of collagen fibers were seen between regenerated muscle fibers (Figure 7B-D) respectively.
Figure 7: Mallory`s trichome stained section of longitudinal section of rat gluteal muscle of group III (eight weeks). (A) Subgroup IIIa (spontaneous healing): extensive collagen (C) fibers in the fibrous tissue at the site of injury and in between muscle bundles (M). (B) Subgroup IIIb (BM-MSCs): minimal amounts of collagen fibers (white arrows) in between the regenerated wavy muscle fibers. (C) Subgroup IIIc (ADSCs): minimal collagen fibers (white arrows) in between the regenerated muscle fibers.
D) Morphometric and statistical results
The mean area percentage of collagen fibers stained by Mallory`s trichrome stain showed significant (p<0.05) increase in all subgroups II and III compared to control group.
Administration of BM-MSCs for ten days (IIb) showed significant (p<0.05) decrease in the mean area percentage of collagen fibers compared to spontaneous healing (IIa) and ADSCs (IIc) subgroups for the same period (ten days). Meanwhile, there was a significant increase compared to BM-MSCs for eight weeks (IIIb).
Administration of ADSCs for ten days (IIc) showed significant (P<0.5) increase compared to BM-MSCs for the same period (IIc) and ADSCs for eight weeks (IIIc). A non-significant decrease was noticed compared to spontaneous healing group of the same period (IIa).
Administration of BM-MSCs for eight weeks (IIIb) showed significant (p<0.05) decrease in the mean area percentage of collagen fibers compared to spontaneous healing (IIIa) group in the same period (eight weeks) and to BM-MSCS for ten days (IIb). Meanwhile, there was a significant increase compared to ADSCs for eight weeks (IIIc).
Administration of ADSCs for eight weeks (IIIc) showed significant (p<0.5) decrease compared to BM-MSCs for the same period (IIIb) and ADSCs for ten days (IIc) (Table 1).
| Group | Mean ± SD area % of collagen fibers (%) | |
|---|---|---|
| Control group | Subgroup Ia | 1.05 ± 0.2 |
| Subgroup Ib | 1.1 ± 0.1 | |
| Ten days experiment | Subgroup IIa | 18.23 ± 0.5* |
| Subgroup IIb | 15.1 ± 0.7*● | |
| Subgroup IIc | 17.4 ± 0.4*○ | |
| Eight weeks experiment | Subgroup IIIa | 21.1 ± 0.5◊ |
| Subgroup IIIb | 8.4 ± 0.3*▲Δ | |
| Subgroup IIIc | 3.1 ± 0.1* ■ |
●Significant increase compared to subgroups IIc& IIIb and
significant decrease compared to IIa
○Significant increase compared to subgroups IIb & IIIc
◊Significant increase compared to all groups
▲Significant decrease compared to subgroups IIb & IIIa
Δ Significant increase compared to subgroup IIIc
■ Significant decrease compared to subgroups IIc, IIIa & IIIb
Table 1: showing the mean ± SD of the area percentage of collagen fibers in different groups
Discussion
Skeletal muscle injury or loss occurs in many clinical situations. Surgical techniques are highly developed and may provide good results for reconstructing muscle function, but surgery is always associated with considerable risks and high costs and even if successful, usually better function at one location is accompanied by impaired function at another location that is less important for the patient [14]. Therefore, scientists are developing techniques in the fields of tissue engineering, cell therapy, and regenerative medicine to aid the regeneration of the muscular-skeletal system [15].
In the present study, healing of experimental skeletal muscle laceration injury was evaluated histologically after injection of two different modalities of stem cell (BM-MSCs and ADSCs).
In the current study, histological examination of untreated experimentally lacerated gluteal muscle after ten days showed that granulation tissue was filling the gap of injury as the muscle fibers retracts during injury [16]. The current study also showed marked mononuclear inflammatory cellular infiltration at the site of injury and in between the remnant muscle fibers. Similar findings were also presented by Moussa who attributed it to the inflammatory response occurring in the early phase after muscle injury [17].
It was reported that disruption of muscle tissue homeostasis, caused by injury, generates sequential events through three main phases. The first phase is degeneration or inflammation phase; occur within the first few days after injury. The initial event is necrosis of the muscle fibers, which is triggered by disruption of local homeostasis and particularly by unregulated influx of calcium through sarcolemma lesions. The second phase is regeneration which includes phagocytosis of damaged tissue, followed by regeneration of myofibers with activation of SCs and formation of connective tissue. The third phase is remodeling in which maturation of regenerated myofibers occurs with recovery of muscle functional capacity. Muscle regeneration, maturation and remodeling usually start during the first 4–5 days after injury, peaks at 2 weeks, and then gradually diminish 3 to 4 weeks after injury [18].
On the other side, histological examination of untreated lacerated muscle after eight weeks showed replacement of the granulation tissue with permanent fibrous tissue at the site of the laceration injury which was noted in H&E and Mallory`s trichrome stained sections. Li [19] reported that after muscle injury, infiltrating lymphocytes, cells of extracellular matrix and myofibroblasts release many growth factors as platelet derived growth factor (PDGF) and transforming growth factor Beta (TGF-B), which subsequently triggers extracellular matrix (ECM) overproduction. This cytokine accelerates the deposition of ECM by increasing the synthesis of ECM proteins on the one hand, while acting to inhibit their degradation on the other.
Shen [20] reported that in the presence of TGF-B, Muscle Derived Stem Cells (MDSCs) and other myogenic cells differentiate into myofibroblasts that produce collagen type I, the major component of fibrotic tissue. Furthermore, Zanotti [21] reported that skeletal muscles fibrosis after injury is attributed to TGF-b1 which is known as one of the most potent profibrogenic factors and regulators of fibrosis development, as it controls ECM synthesis, remodeling and degradation. During muscle repair it can activate the expression of genes that code ECM proteins, such as fibronectin or collagen. On the other hand, TGF-b1 impacts the expression of ECM degrading proteases as, matrix metalloproteinases (MMPs) and their specific inhibitors (tissue inhibitors of MMPs, TIMPs).
In the current study fat cells were noted within the fibrous tissue in spontaneous healing group. Asakura [22] proved that Satellite Cells (SCs) could be stimulated towards both adipogenic and osteogenic lineages in vitro. On other hand Starkey [23] via recombination-based lineage tracing shows that, while SCs can be induced to accumulate lipid, terminal adipogenic differentiation in vitro can’t be achieved. Joe Aw and Uezumi [24,25] isolated a population of cells, fibro-adipogenic progenitors (FAPs), as stem cells in muscle tissue, capable of generating both adipocytes and myofibroblasts in vitro, which do not differentiate in myoblasts in vitro or in vivo.
Consequently, major muscle injuries heal mainly by fibrosis that leads to disturbance of muscle contractile function. This represents a difficult therapeutic dilemma. Therefore, trials are needed to discover a treatment of muscle injury that could promote efficient muscle regeneration.
In the current study, BM-MSCs were used in treatment of laceration injury of right gluteal muscle. Injection of BM-MSCs was concurrent with the procedure of injury and the treated muscles in this group were examined after ten days and after eight weeks from the procedure.
In the current study, general improvement of the state of the muscle was observed especially on the long term (after eight weeks). Site of laceration injury was seen occupied by new regenerating muscle fibers with minimal -almost normal- collagen deposition. Palermo and Shi and Garry [26,27] reported that BM-MSCs promoted the regeneration of muscle fibers by two mechanisms; either all BM-MSCs differentiate to characteristics muscle SCs and then regenerate skeletal muscle fibers, or that a proportion of these cells fuse directly with muscle fibers and that both mechanisms coexist in the same tissue. Sassoli [28] added that BM-MSCs had the ability to contribute to skeletal muscle healing, through the secretion of paracrine factors that supported proliferation and enhanced participation of the endogenous muscle stem cells for regeneration and repair. The release of these paracrine factors could improve the host tissue microenvironment and stimulated the endogenous mechanisms of tissue repair.
In the current study, the ADSCs were also used in treatment of laceration injury of right gluteal muscle, so we could compare between the therapeutic effect of both BM-MSCs and ADSCs. Injection of ADSCs was concurrent with the procedure of injury and the treated muscles in this group were examined after ten days and after eight weeks from the procedure. The ability of ADSCs to differentiate in vitro towards myogenic lineage has been reported by several groups. Culture of ADSCs with myogenic differentiation media has resulted in ADSCs adopting an elongated morphology, like differentiating myoblasts, and expression of early (MyoD1, myogenin) and late (myosin heavy chain) markers of muscle differentiation Zheng. Salgado [29,30] added that ADSCs promote tissue regeneration by secreting cytokines and growth factors that stimulate restoration of normal tissue function or reduce its damage. They reported that molecules secreted by ASCs have a positive effect on the muscles and even the general vitality of cells.
In the present study histological examination of ADSCs treated groups after ten days showed filling the site of laceration injury by less granulation tissue with fewer inflammatory cellular infiltration that extended in between the distorted muscle fibers and congested blood vessels containing RBCs. Similar findings were noted by Moussa [17] "unpublished data". Gonzalez-Rey [31] reported that ASCs are immunoprivileged due to the lack of HLA-DR expression and the proliferation inhibition of activated allogeneic lymphocytes. ASCs also inhibit the generation of pro-inflammatory cytokines, stimulate the production of antiinflammatory IL-10 cytokine and induce the formation of antigenspecific regulatory T cells.
While after eight weeks there was substitution of this granulation tissue by regenerating muscle fibers which were organized in wavy and straight patterns. Liu [32] suggested that not only fusion but also myogenic conversion of ADSCs occurs in vivo since expression of myogenic markers (MyoD, Myogenin, MyHC) from human origin could be detected by RT-PCR with specific primers.
In the current study, differences were noted during culture of both BM-MSCs and ADSCs regarding proliferation rate. ADSCs were seen proliferating at higher rate during both the primary culture and subsequent subcultures. Similarly, Li [33] have compared between MSCs from BM and adipose tissue to determine which type is more effective in cell therapy. They noted that: (1) BMMSCs and ADSCs expanded in hPL-supplemented medium exhibits a homogeneous population with similar fibroblast-like (elongated spindle) morphology.
(2) ADSCs expanded in Human Platelet Lysate (hPL)-supplemented medium have greater proliferative potential than BMMSCs, while there are no significant differences in colony efficiency. (3) BM-MSCs and ADMSCs in hPL-supplemented medium reveal very similar expression patterns of surface markers. (4) BMMSCs expanded in hPL-supplemented medium possess higher capacity toward osteogenic and chondrogenic differentiation compared with ADSCs, while they both have similar adipogenic differentiation potential. (5) There are some differences between BM-MSCs and ADSCs for several secreted proteins, as cytokines, growth factors, and chemokines. (6) ADSCs have more potent immunomodulatory effects than BM-MSCs. Human Platelet Lysate is a human reagent that lacks any risk of secondary effects caused by constituents in culture as fetal bovine serum. So, it increases safety by excluding xenogeneic proteins [34].
Conclusions
Treatment of skeletal muscle laceration injury with either BMMSCs or ADSCs promoted muscle regeneration. However, the result of using ADSCs was better than BM-MSCs. The effect was more prominent after eight weeks than after ten days.
Potential Conflict of Interest
The authors have no conflicting financial interest.
References
- Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, et al. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138: 3647-3656.
- Dumont NA, Bentzinger CF, Sincennes MC, Rudnicki MA (2015) Satellite cells and skeletal muscle regeneration. Compr Physiol 5: 1027-1059.
- Rocheteau P, Vinet M, Chretien F (2015) Dormancy and quiescence of skeletal muscle stem cells. Results Probl Cell Differ 56: 215-235.
- Huard J, Li Y, Fu FH (2002) Muscle injuries and repair: current trends in research. J Bone Joint Surg Am 84-A: 822-832.
- Jarvinen TA, Jarvinen TL, Kaariainen M, Kalimo H, Jarvinen M (2005): Muscle injuries: biology and treatment. Am J Sports Med 33: 745-764.
- Cossu G, Sampaolesi M (2007) New therapies for Duchenne muscular dystrophy: challenges, prospects and clinical trials. Trends Mol Med 13: 520-526.
- Laumonier T, Menetrey J (2016) Muscle injuries and strategies for improving their repair. J Exper Orthop 3: 15.
- Hass R, Kasper C, Böhm S, Jacobs R (2011) Different populations and sources of human mesenchymal stem cells (MSC): Acomparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12.
- Horwitz EM, Gordon PL, Koo WKK, Marx JC, Neel MD, et al. (2007): Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesisimperfecta: implications for cell therapy of bone. Proc Natl Acad Sci U S A 99: 8932-8937.
- Pecanha Pecanha R, de Lima Le Bagno S, Ribeiro MB, Ferreira ABR, Moraes MO, et al. (2012) Adipose-Derived Stem-Cell Treatment of Skeletal Muscle Injury. J Bone Joint Surg Am 94: 609-17.
- Suvarna K, Layton C, Bancroft J (2013) Theory and practice of histological techniques. 7th ed USA: Churchill Livingston.
- MCFarlin K, Gao XK, Liu YB, Dulchavsky DS, Kwon D, et al. (2006) Bone marrow-derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Rep Reg 14: 471-78.
- Cakici C, Buyrukcu B, Duruksu G, Haliloglu AH, Aksoy A, et al. (2013) Recovery of Fertility in Azoospermia Rats after Injection of Adipose-Tissue Derived Mesenchymal Stem Cells: The Sperm Generation. BioMed Research International.
- Liu Juan, Dominik Saul, Kai Oliver Böker, Jennifer Ernst, Wolfgang Lehman (2018) Current Methods for Skeletal Muscle Tissue Repair and Regeneration. BioMed Research International p: 11.
- Alwattar BJ, Schwarzkopf R, Kirsh T (2011) Stem cells in orthopaedics and fracture healing. Bull NYU Hosp Jt Dis 69: 6-10.
- Kaariainen M, Jarvinen T, Jarvinen M, Rantanen J, Kalimo H (2000) Relation between Myofibers and Connective tissue during Muscle injury repair. Scand J Med Sci Sports 10: 332-337.
- Moussa M, Abd El Mohsen A, Hegazy M, Abo El Kasem M (2016) Comparative study on the effect of bone marrow versus adipose tissue derived mesenchymal stem cells on expetimental skeletal muscle injury in rats.
- Tidball JG (2011) Mechanisms of muscle injury, repair, and regeneration. Compr Physiol 1: 2029-2062.
- Li Y, Foster W, Deasy BM, Chan Y, Prisk V, et al. (2004) Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol 164: 1007-1019.
- Shen W, Li Y, Tang Y, Cummins J, Huard (2005) NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol 167: 1105-1117.
- Zanotti S, Saredi S, Ruggieri A, Fabbri M, Blasevich F, et al. (2007) Altered extracellular matrix transcript expression and protein modulation in primary Duchenne muscular dystrophy myotubes. Matrix Biol 26: 615-24.
- Asakura A, Komaki M, Rudnicki M (2001) Muscle satellite cells are multipotential stem cells that exhibit myogenic, osteogenic, and adipogenic differentiation. Differentiation 68: 245-253.
- Starkey JD, Yamamoto M, Yamamoto S, Goldhamer DJ (2011) Skeletal muscle satellite cells are committed to myogenesis and do not spontaneously adopt non myogenic fates. J Histochem Cytochem 59: 33-46.
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, et al. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol 12: 153-63.
- Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol 12: 143-52.
- Palermo AT, Labarge MA, Doyonnas R, Pomerantz J, Blau HM (2005) Bone marrow contribution to skeletal muscle: a physiological response to stress. Dev Biol 279: 336-44.
- Shi X and Garry DJ (2006) Muscle stem cells in development, regeneration, and disease. Genes Dev 20: 1692-1708.
- Sassoli C, Pini A, Chellini F, Mazzanti B. Nistri S (2012) Bone Marrow Mesenchymal Stromal Cells Stimulate Skeletal Myoblast Proliferation through the Paracrine Release of VEGF. PLoS One 7: e37512.
- Zheng B, Cao B, Li GJ (2006) Mouse adipose-derived stem cells undergo multilineage differentiation in vitro but primarily osteogenic and chondrogenic differentiation in vivo. Tissue Eng 12: 1891-1901.
- Salgado AJ, Reis RL, Sousa NJ, Gimble JM (2010) adipose tissue derived stem cells secretome: soluble factors and their roles in regenerative medicine. Curr Stem Cell Res Ther 5: 103-110.
- Gonzalez-Rey E, Gonzalez MA, Varela N, O'Valle F, Hernandez-Cortes P, et al. (2010) Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann Rheum Dis 69: 241-248.
- Liu Y, Yan X, Sun Z, Chen B, Han Q, et al. (2007) Flk-1+adipose- derived mesenchymal stem cells differentiate into skeletal muscle satellite cells and ameliorate muscular dystrophyin MDX mice. Stem Cells Dev 16: 695-706.
- fu Zheng, Guo-bin Zhao, Zhi-jie Ma (2015) Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther 6: 55.
- Lange C, Cakiroglu F, Spiess AN, Cappallo-Obermann H, Dierlamm J, et al. (2007) Accelerated and safe expansion of human mesenchymal stromal cells in animal serum-free medium for transplantation and regenerative medicine. J Cell Physiol 213: 18-26.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences