Coexistence of BehcetÃÆâÃâââ¬Ãâââ¢s Disease and Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Case Report
1Department of Neurology, Military Hospital, Tunis, Tunisia
2Department of Physiology, Medical School, Tunis, Tunisia
- *Corresponding Author:
- Mansour M
Department of Neurology
Military Hospital, Tunis, Tunisia
Tel: +216 55939393
E-mail: mansour.malek18@yahoo.com
Received Date: October 30, 2017; Accepted Date: November 16, 2017; Published Date: November 29, 2017
Citation: Mansour M, Laabidi K, Rachdi A, Riahi A, Zaouali J, et al. (2017) Coexistence of Behcet’s Disease and Chronic Inflammatory Demyelinating Polyradiculoneuropathy: A Case Report. J Nerv Syst Vol.1 No.1:3
Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an acquired polyneuropathy characterized by demyelination of spinal roots and peripheral nerves, with a progressive or relapsing-remitting course, postulated as caused by an immune attack against peripheral myelin. CIPD can be associated with various pathologies, although the precise relationship to the neuropathy may vary. We report the case of an association of Behçet’s disease (BD) and CIDP which is unusual.
Case Report
On the 5th of February 2001, a 50 years old female, with no medical history, was admitted for gait disorder and limb weakness that progressively worsened in more than twenty years. This weakness was preceded by paresthesia in her feet.
Her physical examination revealed symmetrical tetra paresis with generalized areflexia. The muscular strength was 3/5 in proximal and distal muscles of her upper and lower limbs according to the MRC score. She had amyotrophy in her legs and hands with no sensory impairment. The examination of cranial nerves was normal as well as coordination. She reported no bowel or bladder disorder and had no central motor impairment.
Electroneuromyogramm (ENMG) showed features of segmental demyelination: prolonged F-waves latencies, reduced conduction velocities, decreased motor and sensitive amplitudes in all tested nerves, conduction blocks and signs of denervation Tables 1-4.
Table 1. VCS (ENMG results)
| Nerf / 9Sites | Lat. ms | Amp.2-3 µV |
Dist. Cm | Vit.Pic m/s |
|---|---|---|---|---|
| D MEDIAN | ||||
| 1. POIGNET | 1,60 | 17,9 | 9 | 46,2 |
| 2. 4ieme DOIGT | 2,30 | 8,4 | ||
| G MEDIAN | ||||
| 1. POIGNET | 1,60 | 18,3 | 10 | 47,6 |
| 2. 4ieme DOIGT | 2,20 | 7,4 | ||
| 3. COUDE | 2,60 | 3,4 | ||
| D ULNAR | ||||
| 1. POIGNET | ||||
| 2. COUDE | 2,00 | 7,6 | ||
| G ULNAR | ||||
| 1. POIGNET | 2,10 | 3,1 | ||
| D RADIAL | ||||
| 1. POIGNET | 2,00 | 12,8 | ||
| 2. COUDE | 1,90 | 13,0 | ||
| G RADIAL | ||||
| 1. POIGNET | 1.65 | 17,2 | ||
| G SAPHENOUS-EXTERNE | ||||
| CHEVILLE | 3,25 | 6,8 | ||
| 2 | 3,25 | 7,6 | ||
Table 2. VCM(ENMG results)
| Nerf / Sites | Lat. ms | Amp.2-3 µV | Surface mVms | Surf.3-5 mVms | Surf.1-5 mVms | Dist. Cm | Vit m/s |
|---|---|---|---|---|---|---|---|
| D MEDIAN | |||||||
| x1. POIGNET | 5,35 | 1,2 | 3,2 | 2,8 | 6,3 | ||
| 2. COUDE | 14,95 | 0,6 | 1,3 | 2,0 | 3,3 | 27 | 28,1 |
| 3. AISSELE | 19,85 | 0,6 | 1,4 | 1,8 | 3,2 | 9 | 18,4 |
| 4. CREUX SUS CLAV | |||||||
| G MEDIAN | |||||||
| 1. POIGNET | 4,90 | 2,6 | 7,8 | 21,0 | 27,4 | 4 | |
| 2. COUDE | 11,15 | 1,5 | 4,9 | 3,4 | 8,6 | 24 | 38,4 |
| 3. AISSELE | 13,35 | 1,9 | 6,4 | 1,2 | 7,5 | 8 | 36,4 |
| D ULNAR | |||||||
| 1. POIGNET | 4,90 | 0,6 | 2,8 | 10,1 | 11,2 | 4 | |
| 2. COUDE | 16,25 | 0,4 | 0,8 | 1,3 | 2,1 | 22 | 19,4 |
| 3. AISSELE | 22,70 | 0,2 | 1,4 | 10 | 15,5 | ||
| G ULNAR | |||||||
| 1. POIGNET | 3,55 | 2,7 | 7,9 | 4,9 | 13,0 | ||
| 2. COUDE | 10,90 | 1,6 | 4,6 | 3,5 | 8,2 | 22 | 29,9 |
| 3. AISSELE | 14,80 | 1,0 | 2,2 | 1,0 | 3,3 | 9 | 23,1 |
| G COMM PERONEAL | |||||||
| 1.CHEVILLE | |||||||
| 2. TETE PERONE | 45,00 | 0,2 | 0,3 | 0,6 | 0,9 | ||
| D COMM PERONEAL | |||||||
| 1.CHEVILLE | 7,00 | 0,0 | 0,9 | ||||
| 2. TETE PERONE | 17,90 | 0,0 | 0,1 | ||||
| G TIBAL MALLEOLUS | |||||||
| 1.MALLEOLE | 7,30 | 0,6 | 1,7 | 1,1 | 2,8 | ||
| 2.CREUX POPLITE | 7,65 | 0,5 | 1,5 | 1,1 | 2,7 | ||
| D TIBAL MALLEOLUS | |||||||
| 1.MALLEOLE | 16,40 | 0,1 | 0,3 | ||||
| 2.CREUX POPLITE | 9,70 | 0,1 | 0,8 |
Table 3. ONDE F(ENMG results)
| Nerf | Lat M min ms | Lat M moyennems | Lat F min ms | Lat F moyennems | Amp F min mV | Amp F moyenne mV | %F% |
|---|---|---|---|---|---|---|---|
| D ULNAR-DISTAL | 8,20 | 8,25 | 26,25 | 31,81 | 0,1 | 0,1 | 44,4 |
| G MEDIAN-DISTAL | 2,75 | 3,93 | 25,05 | 27,53 | 0,1 | 0,2 | 100 |
| G ULNAR-DISTAL | 5,30 | 22,30 | 30,65 | 30,65 | 0,1 | 0,1 | 16,7 |
Table 4. EMG a I’ aiguille(ENMG results)
| EMG Summary Table | REPOS | Activite |
|---|---|---|
| D.TIB ANTERIOR | Fibrillation | Trace simple |
| D. QUADRICEPS | SE | Intermediate+st |
| D. FIRST D INTEROSS | SE | Intermediate+st |
| D. EXT CARPI R BREV | SE | Intermediate+st |
| G. BICEPS | SE | Intermediate+st |
The cerebrospinal fluid examination didn’t show albumiocytologic dissociation, CSF protein was 0.19 g/l (normal <0.40 g/l) with a cell count of 1 lymphocyte/mm3.
A nerve biopsy performed in 1987 at the onset of symptoms, showed demyelinated and partially remyelinated nerve fibers, endoneurial mononuclear cell infiltration and axon degeneration.
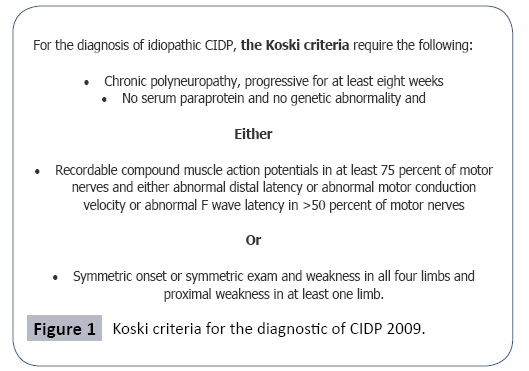
These findings fulfilled criteria for the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy according to the criteria of Koski Figure 1 and the abnormalities found in the ENMG were consistent of a definite PIDC according to the European Federation of Neurological Societies/Peripheral Nerve 2010 task force.
Serology for hepatitis B, C, syphilis and HIV were all negative. She had no cryoglobulinemia. Serum protein electrophoresis didn’t show monoclonal gammopathy. Fasting serum glucose and glycated hemoglobin were normal, sedimentation rate was 15 mm in the first hour and C reactive protein was negative. Screening for auto-antibodies was negative. She was also tested for tumor markers (CA-125, CA 19-9, alpha-Fetoprotein, CA 15-3) which were all absent.
She was treated with corticosteroids: 1 mg/kg/day during one month without improvement. She developed diabetes mellitus so we decided to withdraw steroids.
The decision was to switch to immunoglobulins. She received the first cure of intravenous immunoglobulin in April 2001: 0, 4 g/kg/day during five days with good outcome. We noticed an improvement in her muscular strength from 3/5 to 4/5 using MRC score. Later, a replase occurred in August 2003 so she received another cure of immunoglobulins and we decided to add azathioprine 150 mg/day.
Our patient mentioned that she suffered from recurrent oral and genital aphthae.
The diagnosis of Behcet’s disease was made a few years later in 2003 based on the criteria proposed by The International Study Group on Behcet’s Disease that requires recurrent oral aphthosis and any two of the following lesions (uveitis, genital ulcers, acneiform or folliculitis like lesions, positive pathergy test). Our patient fulfilled all these conditions. She was also tested for HLA-B51 which was positive. Her cerebral MRI didn’t show any abnormalities, spinal cord MRI revealed cervical arthrosis. Therefore; she was treated only using colchicine one tablet per day.
She had two replases requiring the use of immunoglobolins during 5 years. She remains stable.
Discussion
A number of medical conditions may occur contemporaneously with CIPD and have been implicated in its pathogenesis. In our case, it was associated to BD which is rare. Behcet’s disease (BD) is a chronic inflammatory disease which is best described as a multi-system vasculitis. The frequency of neurological involvement, ranges from 5% to 30% and can precede systemic symptoms [1]. Etiopathogensis of BD, like Behcet disease itself, remains to be elucidated.
Central nervous system is frequently involved in BD, but peripheral neuropathy which seen frequently in other vasculitides, is very uncommon in BD [2]. They can present as Guillain-Barre´syndrome, sensorimotor polyneuropathy, CIDP, mononeuritis multiplex or dysautonomic neuropathy [3]. Occasionally, patients with BD have subclinical peripheral nerve involvement with some demyelinating features on EMG studies. In fact, the frequency of neuropathy is higher in the patients with BD when compared with the control subjects [4].
The longer the disease duration; the more is the involvement of peripheral nerves. Full neurological assessment and electrophysiological studies are necessary in the follow up of BD.
The similarities between CIDP and Behcet’s disease: in both diseases immunological mechanisms are probably implicated in the pathogenesis and responsible etiological factors may include a genetically determined predisposition. In fact, both cellular and humoral components of the immune system appear to be involved in their pathogenesis: cellular immunity involvement is supported by evidence of T-cell activation, crossing of the bloodnerve barrier, expression of cytokines, tumor necrosis factor, interferons, and interleukins. Humoral immunity is implicated by the demonstration of immunoglobulin and complement deposition on myelinated nerve fibers. This hypothesis is strongly supported by the fact that the vast majority of patients improve after receiving immune therapies.
Besides, in vasculitic neuropathies such as BD, it is possible that after a damage of blood vessels, T cells are attracted nonspecifically into the nervous tissue. Clogging of the blood vessels causes ischemia notably in the nerves which can cause the axons to degenerate.
Similar to BD, pathologic investigations in CIDP show mononuclear cell infiltration of monocytes/macrophages and less commonly T lymphocytes into peripheral nerves, endoneurial inflammation and nerve demyelination mediated by complement and antibodies direct against antigenic components of the myelin sheath [5].
BD is not probably a predisposing risk factor for CIPD compared with the general population. Clinical confusion arises when some patients with BD develop a severe, progressive symmetric neuropathy that is out of proportion to the severity of the BD.
In such cases, it can be difficult to know whether these cases should be classified as CIDP in a BD patient and treated with standard immune therapy, or a neuropathy as a complication of BD.
Until clinical trials can study this specific group of patients, it is difficult to draw firm conclusions.
Conclusion
CIPD is a cause of disability and more than half of affected people cannot walk unaided when symptoms are at their worst. Therefore, the diagnosis and an exhaustive assessment of associated pathologies should be made precociously. Finally, we want to stress the point that BD can be associated to CIPD so we should consider it in our investigations [6].
References
- Talarico R, D’Ascanio A, Figus M, Stagnaro C, Ferrari C, et al. (2012) Behcet's Disease; features of neurological involvement in a dedicated centre in Italy. Clin Exp Reumatol 72: 69-72.
- Atasoy HT, Tunc TO, Unal AE, Emre UM, Koca RM, et al. (2007) Peripheral nervous system in patients with Behcet disease. Neurologist 13: 225-230.
- Briol A, Ulkaten S, Kocak M, Erkek E (2004) Peripheral neuropathy in Behcet ‘s disease. J Dermatol 31: 455-459.
- Akbulut L, Gur G, Bodur H, Alli N, Borman P (2007) Peripheral neuropathy in Behcet disease: an electroneurophysiological study. Clin Rheumatol 26: 1240-1244.
- Ghorbel B, Ibnalhadj Z, Zouari M, Nagi S, Khanfir M, et al. (2005) Behcet’s disease associated with peripheral neuropathy. Rev Neurol (Paris) 161: 218-220.
- Eun MY, Kang CH, Kim BJ (2011) Tibial neuropathy associated in Behcet’s disease. Am J Phys Med Rehabil 90: 432-433.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences