ISSN : 2574-0431
Synthesis and Catalysis: Open Access
Catalytic Production of Syngas by Methane Reforming
Particles and Catalysis Research Group, School of Chemical Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
- *Corresponding Author:
- Hamidreza Arandiyan
School of Engineering
The University of Hull
Cottingham Road, Hull HU6 7RX, UK
Tel: +61 2 9385 1000
E-mail: h.arandiyan@unsw.edu.au
Received Date: March 15, 2017; Accepted Date: March 16, 2017; Published Date: March 17, 2017
Citation: Arandiyan H, Wang Y. Catalytic Production of Syngas by Methane Reforming. Synth Catal. 2017, 2:1.
The research on methane reforming has recently gained considerable attention and proved the scientific interest as a way of producing hydrogen through the generation of synthesis gas (mixed gas of hydrogen and carbon monoxide). The conversion of syngas can be conducted by steam reforming and dry reforming, using H2O/CH4 or CO2/CH4 as feed gas respectively. Dry reforming of methane (DRM) process can achieve a higher CO/H2 product ratio (3:1) than that of steam reforming (1:1). Additionally, DRM has also received attention from the environmental consideration since the emission of methane and carbon dioxide into the atmosphere causes undesirable effect on global warming through the greenhouse effect, and these harmful gases can be simultaneously converted into useful syngas [1].
Ni and Co catalysts were the first studied for DRM by Fischer and Tropsch in 1928. However, these catalysts were observed to be severely deactivated due to carbon deposition [2]. Since then, the researchers dedicated to pursue the high efficient and coke-resistant catalysts for DRM and numerous materials like Ni, Co, Fe and noble metal (Pt, Rh, Pd, Ir) have been reported to be active for DRM [3-5]. Precious metals are proved to be less sensitive to coking as compared to Ni and the addition of second metals (alkali earth and rare earth metals) is of importance to reduce carbon deposition. Arandiyan et al. [6], synthesized the tri-metallic perovskite mixed oxides LaNixFe1-xO3 for the reforming of CH4 to syngas and found the substitution of Fe in Ni perovskite catalyst affect the product rate and promote the stability. They also discovered that introducing O2 with the feed gas flow can accelerate the decomposition of carbon and thus prevent the catalytic deactivation. The effect of modification with alkali metals (M=Li, Na, K) in MxLa1-xNi0.3Al0.7O3-d was investigated for DRM to produce syngas [1]. The Na incorporation in La site in the perovskite enhanced the activity efficiency while the K and Li substitution increased the reduction temperature and decreased the conversion rate of CH4 at the same temperature comparing with LaNi0.3Al0.7O3-d catalyst. However, the substituted catalysts with alkaline metals showed significant stability after 15 h on stream reaction, maintain their activity and selectivity in the production of syngas. In addition, the addition of Sr and Ca of La also showed specific influence on the activity and stability for DRM [7].
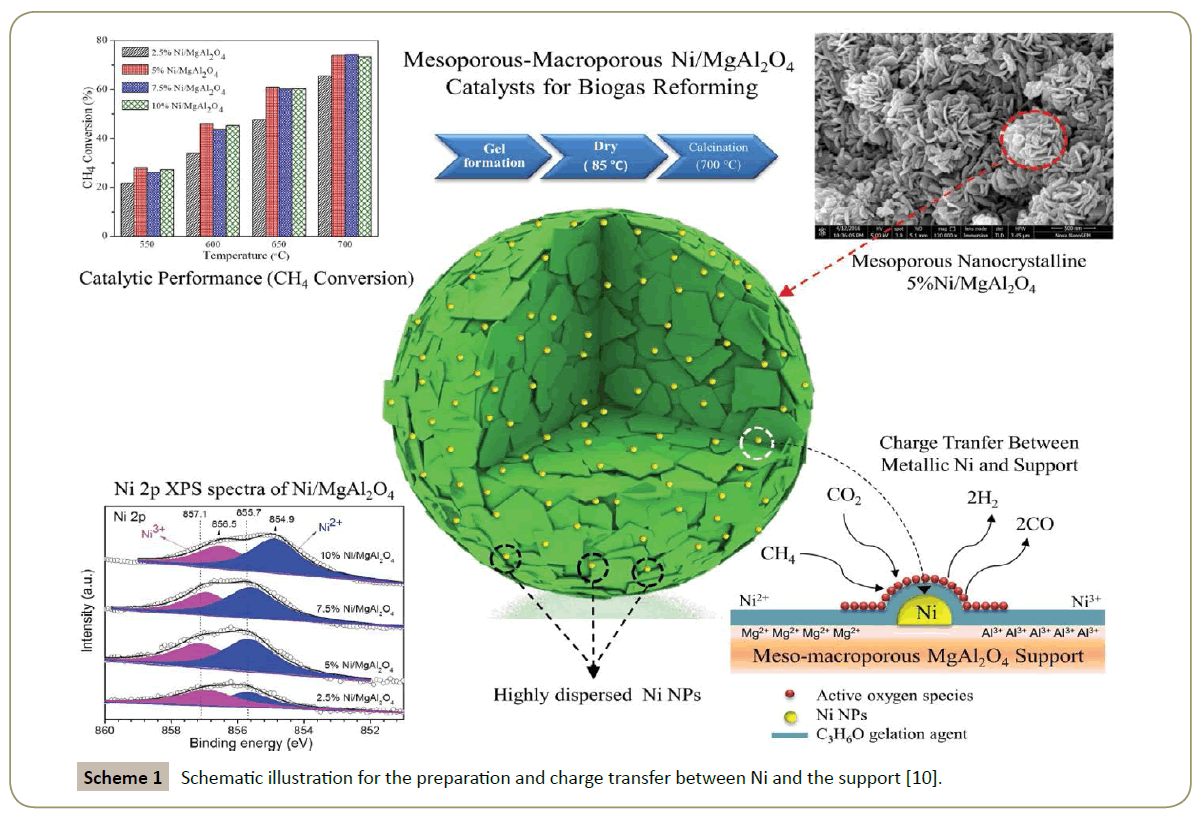
In his recent study, Arandiyan and co-workers using a facile and simple propylene oxide assisted gelation agent route to successfully synthesize mesoporous nanocrystalline MgAl2O4 [8,9]. The obtained spinel catalysts has large surface area in the range of 253-302 m2/g and narrow single modal pore size distributions in the mesopore region which promote the catalytic performance for CH4 reforming and resist against the carbon formation. Alternatively, they dispersed Ni nanoparticles on this mesoporous MgAl2O4 spinel as shown in Scheme 1 and obtained an enhancement on the syngas formation from DRM [10]. The exchange of Mg2+ by Ni2+ was observed at the low Ni loading sample (5 wt%) and this appropriate partial Ni substitution in the MgAl2O4 spinel was believed to play a role in the elevated catalytic performance.
Scheme 1: Schematic illustration for the preparation and charge transfer between Ni and the support [10].
In the future study on DRM catalyst, the inevitable deposition of surface carbon is still a major issue. Using basic support and appropriate catalyst activation/calcination procedure, promoter to metal ratio, metal precursor is the ideal path to avoid deactivation and stable performance of the catalyst for DRM.
References
- Khalesi A, Arandiyan HR, Parvari M (2008) Production of Syngas by CO2 Reforming on MxLa1-xNi0.3Al0.7O3-d (M=Li, Na, K) Catalysts. IndEng Chem Res 47: 5892-5898.
- Pakhare D, Spivey J (2014) A review of dry (CO2) reforming of methane over noble metal catalysts. Chem Soc Rev 43: 7813-7837.
- Rezaei M, Alavi SM, Sahebdelfar S, Xinmei L, Qian L, et al. (2007) CO2-CH4 Reforming over Nickel Catalysts Supported on Mesoporous Nanocrystalline Zirconia with High Surface Area.Energy & Fuels 21: 581-589.
- Rezaei M, Alavi SM, Sahebdelfar S, Yan ZF (2006) Nanocrystalline Zirconia as Support for Nickel Catalyst in Methane Reforming with CO2. Energy & Fuels 20: 923-929.
- Arandiyan H, Peng Y, Liu C, Chang H, Li J (2014) Effects of noble metals doped on mesoporous LaAlNi mixed oxide catalyst and identification of carbon deposit for reforming CH4 with CO2. J Chem TechBiotechnol 89: 372-381.
- Arandiyan H, Li J, Ma L, Hashemnejad SM, Mirzaei MZ, et al. (2012) Methane reforming to syngas over LaNixFe1-xO3 (0 = x = 1) mixed-oxide perovskites in the presence of CO2 and O2. J IndEng Chem 18: 2103-2114.
- Khalesi A, Arandiyan HR, Parvari M (2008) Effects of Lanthanum Substitution by Strontium and Calcium in La-Ni-Al Perovskite Oxides in Dry Reforming of Methane. Chinese J Catal 29: 960-968.
- Habibi N, Wang Y, Arandiyan H, Rezaei M (2017) Low-temperature synthesis of mesoporous nanocrystalline magnesium aluminate (MgAl2O4) spinel with high surface area using a novel modified sol-gel method. AdvPowder Technol.
- Habibi N, Arandiyan H, Rezaei M, Mesoporous (2016) MgOAl2O3 nanopowder-supported meso-macroporous nickel catalysts: a new path to high-performance biogas reforming for syngas. RSC Adv 6: 29576-29585.
- Habibi N, Wang Y, Arandiyan H, Rezaei M (2016) Biogas Reforming for Hydrogen Production: A New Path to High-Performance Nickel Catalysts Supported on Magnesium Aluminate Spinel. ChemCat Chem 8: 3600-3610.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences