Acute Type B Aortic Dissection Presenting as Acute Abdomen
Liang-Ying Ke1, Hong-Jie Jhou1, Hui-Chen Yu2, Chun-Chen Chen3 and Ying-Fu Chen2,3,4*
1School of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
2Division of Cardiovascular Surgery, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
3Division of Cardiovascular Surgery, Tainan Sin-Lau Hospital, Tainan, Taiwan
4Graduate Institute of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- Corresponding Author:
- Ying-Fu Chen
Division of Cardiovascular Surgery
Department of Surgery, Kaohsiung Medical University Hospital
Kaohsiung Medical University, 100, Shih-Chuan 1st Rd, Kaohsiung 807, Taiwan
Tel: 886-7-312-1101 (ext. 5801)
Fax: 886-7-312-7056
E-mail: yfchen@kmu.edu.tw
Received date: May 2, 2018; Accepted date: May 16, 2018; Published date: May 25, 2018
Citation: Ke LY, Jhou HJ, Yu HC, Chen CC, Chen YF (2018) Acute Type B Aortic Dissection presenting as Acute Abdomen. J Anaesthesiol Crit Care. Vol 1 No.2:10
Copyright: © 2018 Ke LY, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
This report presents a surgical repair of a complicated acute type B aortic dissection in a 42-year-old male who suffering from acute abdominal pain initially and the postoperative course was complicated with chylothorax. Acute aortic dissection is a life-threatening vascular emergency and the anterior chest pain radiating to the back is the typical pattern when it attacks suddenly. However, epigastralgia or diffused abdominal pain could be experienced when abdominal branch vessels were involved. Here, we review the literature on acute aortic dissections complicated by involvement of abdominal branch vessels, causing visceral malperfusion or mesenteric ischemia. Vital organ malperfuion, including major cerebral malperfusion, coronary malperfusion and mesenteric ischemia, have been identified as independent risk of in-hospital mortality in several large study enrollments. Despite it still remains debates of strategic and therapeutic options to correct these defects, in-time evaluations and proper examinations should be performed promptly to lower the misdiagnosis rate and reduce the extremely high in-hospital mortality.
Keywords
Acute aortic dissection (AAD); Abdominal pain; Mesenteric ischemia; Superior mesenteric artery (SMA)
Introduction
Acute aortic dissection (AAD), an aortic emergency, is a potentially lethal disease. The incidence of AAD is infrequent, about 3.5-6.0 per 100,000 patients-years in the general population, but a rapid diagnosis and immediate management are critical for salvage [1]. Higher mortality may occur when the perfusion to vital organ or tissue is decreased due to branchvessel occlusion [2]. We have been reported a case of a middleaged male presenting bilateral hemathoraces as a rare clinical presentation for acute type B aortic dissection [3]. The patient also experienced three episodes of severe epigastralgia preoperatively and he was rapidly arranged open surgery after the diagnosis confirmed. Herein, we now report the case of severe epigastralgia caused by acute aortic dissection and discuss this critical issue in depth.
Clinical Scenario
A 42-year-old man presented to emergency department with three episodes of acute cramping pain over upper abdomen within six days in April, 2004. He had a history of hepatitis B and hypertension without regular medication control. Discharged after pain relief in the first emergency care and brought back again 20 hours later, being resolved by glycine enema. The esophagogastroduodenoscopy (EGD) was arranged on the next day for detailed survey.
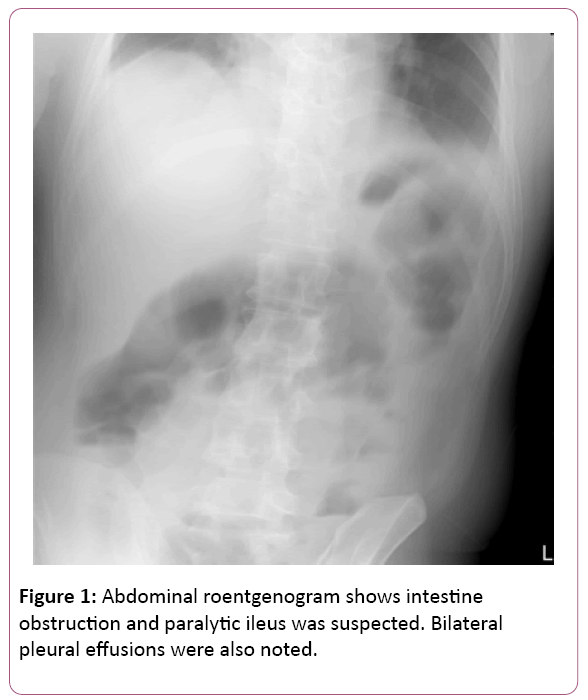
The EGD findings revealed superficial gastritis in the body portion and a grade A reflux esophagitis. Nevertheless, the patient rushed to the emergency room again four days later and complained about severe, acute epigastralgia accompanied with diarrhoea, cold sweating and decreasing urine output. The abdominal plain film was initially performed showing paralytic ileus and intestinal obstruction was suspected (Figure 1). Thus, he was suggested admitting to the gastrointestinal ward for further management.
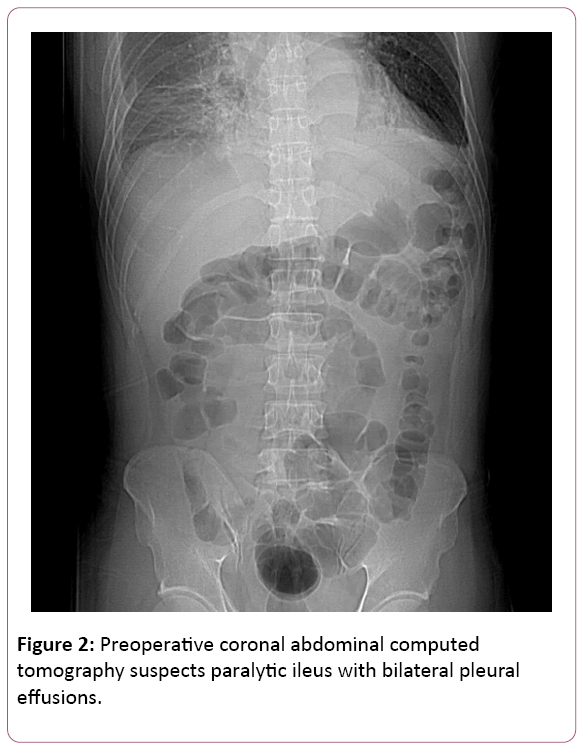
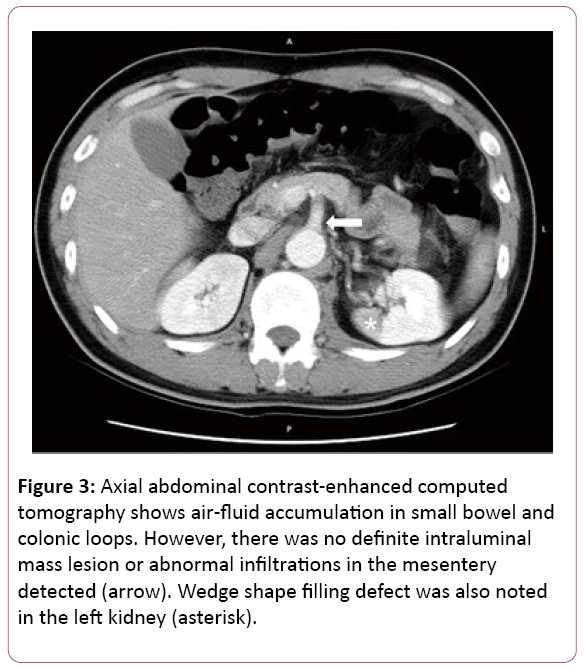
All at once, acute chest pain attacked and radiated to his back, right chest, left shoulder and chin few hours later. Initial examinations were normal and EKG revealed ST elevation at V2. CK-MB and Troponoin-I were both in normal range, disclosing 1.7 ng/mL and below 0.04 ng/mL. Chest X-ray showed mediastinal widening, cardiomegaly, dilated aorta and bilateral pleural effusion. Succeeding chest computed tomography confirmed the diagnosis of acute type B aortic dissection; left renal artery involvement and suspected impending aortic rupture (Figures 2 and 3).
Figure 3: Axial abdominal contrast-enhanced computed tomography shows air-fluid accumulation in small bowel and colonic loops. However, there was no definite intraluminal mass lesion or abnormal infiltrations in the mesentery detected (arrow). Wedge shape filling defect was also noted in the left kidney (asterisk).
He was transferred to the intensive care unit afterward giving close attention to the vital signs, paging anesthesiologist, cardiologist and cardiovascular surgeon subsequently for evaluations expeditiously. Echocardiogram indicated normal left ventricular systolic function and without aortic regurgitation. ASA Class IV was assessed basing on American Society of Anesthesiologists Classification (ASA) and emergency operation was arranged.
The blood pressure revealed 113/51 mmHg during surgical preparation and atropine, demerol, primperan plus cefamezine were administered for premedication then fentanyl, recofol and esmeron for anesthetic induction. The operation began under general anesthesia and intraoperative transesophageal echocardiography (TEE) implied normal cardiac wall motion. 1500 mL of blood was drained from right hemithorax when a chest tube was inserted. Left posterolateral thoracotomy was applied, a massive hematoma was found around the aortic arch and the whole descending thoracic aorta after opening the left hemithorax. An 8-mm, sealed, woven Dacron side branch was anastomosed to the main aortic Dacron prosthesis by end-toside fashion during the period of systemic cooling phase for later proximal perfusion. The intimal tear was identified on the lesser curvature of the distal aortic arch following aortotomy and the proximal aorta was transected just proximal to the intimal tear. The inner and outer glutaraldehyde-treated Teflon strips bolsters were sewn into each, proximal and distal, transected aortic end. The precoated Dacron graft (Hemashield Platinum, Woven Double Velour, Boston Scientific Corp, Wayne, NJ, USA) was anastomosed to the proximal aortic end. Lastly, the distal aortic anastomosis was performed to the distal third of the descending aorta. Unfortunately, the postoperative course was complicated by left massive chylothorax. The severed thoracic duct was ligated via re-thoracotomy at eleventh postoperative day because medical management of chylothorax has failed to reduce chyle flow. He got over it and discharged smoothly after 29-day admission.
To date, the patient keeps regular follow-up at our cardiovascular outpatient department for almost 14 years without further cardiovascular event. Of note, unfortunately, the only patient’s sibling died from acute type A aortic dissection three months ago.
Discussion
Mostly, acute aortic dissection patients presented with the impetuous, sharp pain on anterior chest along with radiating dorsalgia or not. It might be less frequent below the diaphragm and could be insidious or exaggerated because of static or dynamic, even mixed type, of branch-vessels occlusion especially involving celiac trunk or superior mesenteric artery causing sudden, transient deterioration of the organ circulation [4,5]. About 20% of type A and half of type B victims suffered bellyache in previous studies [6]. However, epigastralgia and abdominal pain were both equivocally expressed by either patients or clinicians. As we know, there were only few contemporary series reported regarding the relationship between acute aortic dissections and acute abdomen, thus, that are worth a discussion among us.
Precisely to localize the origin of abdominal pain seems not easy because of visceral sensations are typically referred to nonvisceral somatic tissues. Second-order spinal dorsal horn neurons receive visceral and somatic inputs which is the cornerstone of referred pain, thus, it might be confusing the patients or clinicians when damage happens in abdominal cavity [7]. Under the acknowledgements of embryonic development, in short, digestive system rotating, evolving into three parts in the period of fetal developments with complex nerve innervations. Pain typically implodes in epigastric region when foregut structures from stomach to the second part of duodenum are involved. On the other hand, pain from lesions of midgut structures including distal duodenum, small intestine, appendix, ascending and proximal two-third of transverse colon often refers in the periumbilical area [8].
Bauersfeld [9] stated fifteen dissecting aneurysm patients in the fifth decade of last century and eight of whom complained about abdominal pain. It is noteworthy that a female octogenarian complained epigastralgia with vomiting and nausea for 3 weeks which was revealed similar presentation as our patient. No abnormal findings were found in regular examinations except the abdominal flat plate indicated dilated loop of bowel of this old woman. Partial intestinal obstruction was impressed initially and the final diagnosis was dissecting aneurysm of superior mesenteric artery. La’mentedly, apart from our lucky guy, she died from gangrene of the bowel due to thrombus occluded the artery.
Persistent epigastralgia with increasing acute phase proteins and lactate dehydrogenase are indicators of intimal tear involving celiac trunk or its proximal branches which might be resulted in spleen infarction, gastritis or even unusual acute pancreatitis. All of which has been reported showing both elevated serous and pancreatic-specific amylase [10,11]. Instead, the term “acute abdomen” seems more comprehensive and many associated symptoms could obscure the presentation when hypoperfusion is evoked by abrupt aortic dissection and might impede judgements. Despite the good sensitivity and specificity of multidetector computed tomography (MDCT) and CT angiography, affected mesenteric artery may mimic mechanic obstruction or disguise from benign infection or inflammation to malignancy in image studies. Thus, findings like embolus or thickening enteric wall should not easily rule out aortic dissection [12]. In recent study, investigators proclaimed that the optimized duplex ultrasonography (DUS) protocol might be feasible to apply to detect descending thoracic aortic pathology while it is not traditionally use in acute assessment [13]. Five to seven percent of incidence complicated by visceral ischemia and a high mortality rate in statistic significant have been reported in both Type A and Type B lesions [14]. On multivariable logistic regression model in recent IRAD database, mesenteric ischemia was identified as the predictor of in-hospital mortality with the odds ratio of 8.05 in Type A patients and 8.64 in Type B group [15]. Furthermore, the comparison between operative risk and stratified groups in Penn classification has been advocated by Augoustides and associates implying the severity of ischemic vital organs [16,17]. Chien et al. [2] and Li et al. [18] further modified the original grouping of Penn classification, pointed out that critical organ-specific ischemia, comprising major cerebral malperfusion, mesenteric ischemia and coronary malperfusion, should be distinguished into a specific subgroup which carries a much higher risk of in-hospital mortality compared to other branch-vessel ischemia and is a force to be reckoned with in the ischemic presentation of patients with acute aortic dissection. Statistics indicated that malperfusion is the cause of death second only to aortic ruptures in patients with acute Type B aortic dissections [19]. Hence, if clinical symptoms such as syncope or unequal femoral pulse were found in patients with acute, severe abdominal pain in emergency department, overwhelming history taking, holistic evaluations and examinations should be established on the time for considerate potential intra-abdominal vascular calamity including aortic dissection or ruptured abdominal aortic aneurysm (AAA), making timely intervention and blown underreport symptoms especially in the elderly. [8] Multiple linear regression analysis from International Registry of Acute Aortic Dissection (IRAD) implied a delayed diagnosis for definitive treatment in acute aortic dissection deteriorating the grave prognosis [20]. Di Eusanio and the colleagues [21] also suggested that immediate surgical intervention with or without percutaneous treatment restoring mesenteric perfusion still has 2 folds in-hospital mortality rate but better long-term survival. Kamman et al. [22] proposed a treatment algorithm based on their clinical experiences at University of Michigan and the former studies about visceral and mesenteric ischemia although the current opinion of the optimal surgical strategies for acute aortic dissection complicated with mesenteric ischemia is still inconsistent. To sum up, mesenteric ischemia is a “silent killer” in patients with acute aortic dissection if we are missing the boat.
In our patient, mesenteric ischemia was suspected based on symptoms and the findings of abdominal roentgenogram. Nevertheless, the exact pathophysiological mechanism of it could not be assured because the abdominal CT interpreted even by experienced radiologist did not document intimal tear involved the mesentery artery. Déjà vu, Fukunaga and coworkers hypothesized that thoracic aortic dissection might trigger abnormal hyperactivity of sympathetic nervous systems leading to mesenteric vasospasm in their reported victim, a 78- year-old woman with acute type A aortic dissection presenting paralytic ileus [5,23].
Due to the spectrum of clinical manifestations is broad and mercurial, the initial diagnosis relies on only clinicians’ intuitions not always without a single miss. Asouhidou et al. [24] demonstrated that one third (15 of 49) of the patients with or without typical presentation, misdiagnosing initially in a retrospective study. Paralytic ileus may not the common symptom of acute aortic dissection and the exact pathophysiology has not yet been completely clarified. Nevertheless, non-occlusive mesenteric malperfusion should also be highly considered in such patients to avoid misdiagnosis.
Conclusion
Acute type B aortic dissection presenting as acute abdomen revealed in various clinical courses but might not always be dreadful, of note, it is prone to be escaped from detection. However, mortality rate is skyrocketing if the pain is triggered by acute aortic dissections even involves supra-mesenteric artery, creating the confusion to clinicians. Hence, serial, careful examinations and emergency remedy should be done for those who is complicated by celiac trunk or SMA tangle in order to have better therapeutic outcome.
References
- Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, et al. (1993) Practice guidelines for management of the difficult airway. A report by the American Society of Anesthesiologists task force on management of the difficult airway. Anesthesiology 78: 597-602.
- Caplan RA, Benumof JL, Berry FA, Blitt CD, Bode RH, et al. (2003) Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists task force on management of the difficult airway. Anesthesiology 98: 1269-1277.
- Benumof JL (1991) Management of the difficult adult airway. With special emphasis on awake tracheal intubation. Anesthesiology 75: 1087-1110.
- Tachibana N, Niiyama Y, Yamakage M (2015) Incidence of cannot intubate-cannot ventilate (CICV): Results of a 3 year retrospective multicenter clinical study in a network of university hospitals. J Anesth 29: 326-330.
- Combes X, Jabre P, Margenet A, Merle JC, Leroux B, et al. (2011) Unanticipated difficult airway management in the prehospital emergency setting: Prospective validation of an algorithm. Anesthesiol 114: 105-110.
- Davies S, Ananthanarayan C, Castro C (2001) Asymptomatic lingual tonsillar hypertrophy and difficult airway management: A report of three cases. Can J Anaesth 48: 1020-1024.
- Cook TM, Woodall N, Frerk C (2011) Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 1: Anesthesia. Br J Anaesth 106: 617-631.
- Asai T (2012) Videolaryngoscopes: Do they truly have roles in difficult airways? Anesthesiol 116: 515-517.
- Shiga T, Wajima Z, Inoue T, Sakamoto A (2005) Predicting difficult intubation in apparently normal patients: A meta-analysis of bedside screening test performance. Anesthesiol 103: 429-437.
- Samsoon GL, Young JR (1987) Difficult tracheal intubation: A retrospective study. Anesth 42: 487-490.
- Cook TM, Woodall N, Harper J, Benger J (2011) Major complications of airway management in the UK: Results of the Fourth National Audit Project of the Royal College of Anaesthetists and the Difficult Airway Society. Part 2: Intensive Care and Emergency Department. Br J Anaesth 106: 632-642.
- Asai T, Liu EH, Matsumoto S, Hirabayashi Y, Seo N, et al. (2009) Use of the Pentax-AWS in 293 patients with difficult airways. Anesthesiol 110: 898-904.
- Griesdale DE, Liu D, McKinney J, Choi PT (2012) Glidescope(R) video-laryngoscopy versus direct laryngoscopy for endotracheal intubation: A systematic review and meta-analysis. Can J Anaesth 59: 41-52.
- Asai T (2013) Strategies for difficult airway management-the current state is not ideal. J Anesth 27: 157-160.
- Apfelbaum JL, Hagberg CA, Caplan RA, Blitt CD, Connis RT, et al. (2013) Practice guidelines for management of the difficult airway: an updated report by the American Society of Anesthesiologists task force on management of the difficult airway. Anesthesiol 118: 251-270.
- Henderson JJ, Popat MT, Latto IP, Pearce AC (2004) Difficult Airway Society guidelines for management of the unanticipated difficult intubation. Anesthesia 59: 675-694.
- Saito T, Liu W, Chew STH, Ti LK (2015) Incidence of and risk factors for difficult ventilation via a supraglottic airway device in a population of 14 480 patients from south-east Asia. Anesthesia 70: 1079-1083.
- Liu EH, Asai T (2015) Cannot intubate cannot ventilate—focus on the 'ventilate'. J Anesth 29: 323-325.
- Adnet F (2000) Difficult mask ventilation: an underestimated aspect of the problem of the difficult airway? Anesthesiol 92: 1217-1218.
- Ramachandran SK, Mathis MR, Tremper KK, Shanks AM, Kheterpal S (2012) Predictors and clinical outcomes from failed Laryngeal Mask Airway Unique: a study of 15,795 patients. Anesthesiol 116: 1217-1226.
- Randell T (1996) Prediction of difficult intubation. Acta Anaesthesiol Scand 40: 1016-1023.
- Kamman AV, Yang B, Kim KM, Williams DM, Michael Deeb G, et al. (2017) Visceral Malperfusion in Aortic Dissection: The Michigan Experience. Semin Thorac Cardiovasc Surg 29: 173-178.
- Fukunaga N, Konishi Y, Matsuo T, Saji Y, Koyama T (2017) Paralytic ileus as the first presentation in type A acute aortic dissection. J Med Invest 64: 286-287.
- Asouhidou I, Asteri T (2009) Acute aortic dissection: Be aware of misdiagnosis. BMC Res 2: 25.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences