
Crystallography 2018
Structural Chemistry & Crystallography Communication
ISSN: 2470-9905
Page 21
June 04-05, 2018
London, UK
3
rd
Edition of International Conference on
Advanced Spectroscopy,
Crystallography and Applications
in Modern Chemistry
T
he role of halogens in racemic 4-hal-4-butyl (n-octyl)-1-
phenyl derivatives (hal: F, Cl, Br), of the cyclic pyrazoline-
(1,3)-diones in the solid state and in solution was determined
[1]. Noncovalent interactions are observed for the F, Cl and Br
derivatives between the halogen atom and the hydrogen atom
of the nitrogen of the pyrazolidine ring, water hydrogens that
interact either with the halogen atoms or with the carbonyl
oxygen atoms very different from the none-halogenated
pyrazoline-diones [2-5]. The 3d and 2d structures are stabilized
by - and - interactions, intermolecular distances, and apolar
forces between adjacently stacked phenyl rings. However, the
R-or S-enantiomers or their water-stable complexes with Zn-
meglumine did not racemizes in aqueous dispersions [1,3].
Small-angle-, wide-angle x-ray scattering experiments, and
molecular simulation reveal similar solution structure factors,
S(Q), in the solid state and in solution [6,7]. The planes and
their periodicities of the crystalline phases are preserved in
the aqueous solution phase. There is also hydrogen bonding
formed in the racemic and the R-enantiomeric n-octyl-1-
phenyl-1-Cl-pyrazoline-(1,3)-dione between the hydrogens
of the water molecules and the halogens of the pyrazolidine
ring: Cl forms a hydrogen bond to the water hydroxy group
of a neighbouring molecule, which is hydrogen bonded to
the chlorine of another molecule forming a 1-dimensional
hydrogen-chloride bond network differently from hydrated
cationic lipids or their polymorphs [8,9]. The n-octyl pyrazolidine
approximant forms micelles in aqueous dispersions that self-
assemble into quasicrystalline structures. The small-angle
X-ray scattering experiments and the selected area electron
diffraction pattern of thin films suggest that the micelle FCC
phase transforms into a colloidal quasicrystalline phase with
12-fold symmetry that proceed through rearrangements of the
micelles in the (111) layers of the FCC phase. The differences
of the halogenated cyclic and non-cyclic pyrazoline diones are
related to biochemical changes in anti-inflammatory activities.
The n-octyl compound reveal antimicrobial and antiviral
(influenza) activities but no anti-inflammatory or analgesic
activities.
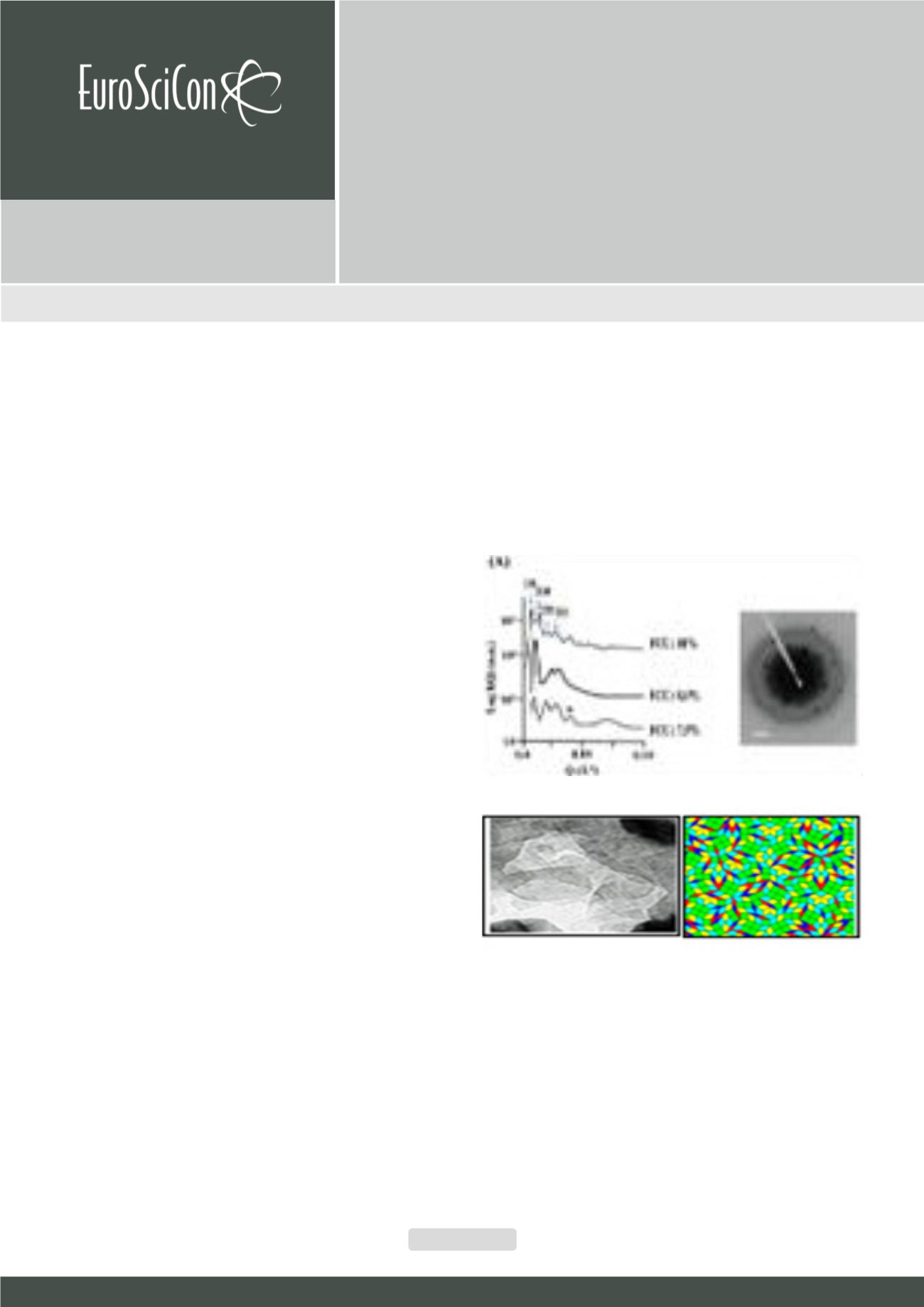
Fig.1.
(Left) SAXS curves for isotropic (R,S)-4-n-octyl-4-Cl-1-phenyl-3, 5-pyr-
azolidine-dion samples for different concentrations (20°C). For the 19%
(w/w), 8.0% (w/w) and 7.5% (w/w) solutions the Q-positions of the observed
structure peaks of the scattering curves can be simulated with and a = 50.0
Å. The broad reflection at Q = 0.035 Å-1 correspond to the 11110 reflections
of a face-centerd cubic lattice (Fm3m) of the crystalline phase (20°C). The
scale bar 30 nm. (Middle) HRTEM image of quasicrystals obtained from
a 7.5% (w/w) (R,S)-n-octyl-Cl-phenyl-3,5-pyrazolidine-dion solution. (Right)
Tiling pattern generated from the tessellation graphic (middle) applying tri-
angles and squares for two Archimedean (3342) materials: three domains
in tortoise, red and blue assigned to the p4g and two domains, green and
yellow for the p6m approximants
STEREOCHEMISTRY AND ANTI-INFLAMMATORY INHIBITION: ASYMMETRY,
AND COMPLEXES OF 4-HALOGENATED MOFEBUTAZONES DERIVATIVES
Henrich H. Paradies
1
and
Hendrick Reichelt
2
1
Jacobs-University, Germany
2
Salford University, United Kingdom
Henrich H. Paradies et al., Struct Chem Crystallogr Commun 2018, Volume 4
DOI: 10.21767/2470-9905-C1-005
















