
Crystallography 2018
Structural Chemistry & Crystallography Communication
ISSN: 2470-9905
Page 50
June 04-05, 2018
London, UK
3
rd
Edition of International Conference on
Advanced Spectroscopy,
Crystallography and Applications
in Modern Chemistry
A
major challenge in drug discovery is the identification of
chemical moieties that specifically interact with a particular
proteintarget.Traditionally,thiswasaddressedbyHighThroughput
Screening (HTS) however, recently “Fragment Screening” has
become increasingly popular. In a Fragment Screen a set of
small molecules (“fragments”), typically with MW < 300 Da and
with low affinities, are evaluated for specific interaction with a
target. Crystallography/X-ray diffraction shows not only whether
a fragment binds to the protein but also where and how the
binding occurs and is therefore the favored screening method [1-
3]. Hit-fragments are subsequently chemically modified in several
optimization/screening cycles until a high affinity lead structure
is obtained (figure 1). Since such a fragmented approach allows
screening of broader chemical space compared to large, distinct
libraries, the hit rates of Fragment Screens are believed to be 10-
1000x higher than those in traditional HTS [4].
The *Frag Xtal Screen* is a unique Fragment Screen designed for
direct crystallographic screening: 96 different fragments, selected
for high chemical diversity, high solubility and for being validated
crystallographic hits of several protein targets, are spotted onto
the wells of a crystallization plate. This screening plate is ready-
to-use for crystal soaking experiments and offers an easy entry
to fragment-based lead discovery (FBLD) by crystallographic
screening.
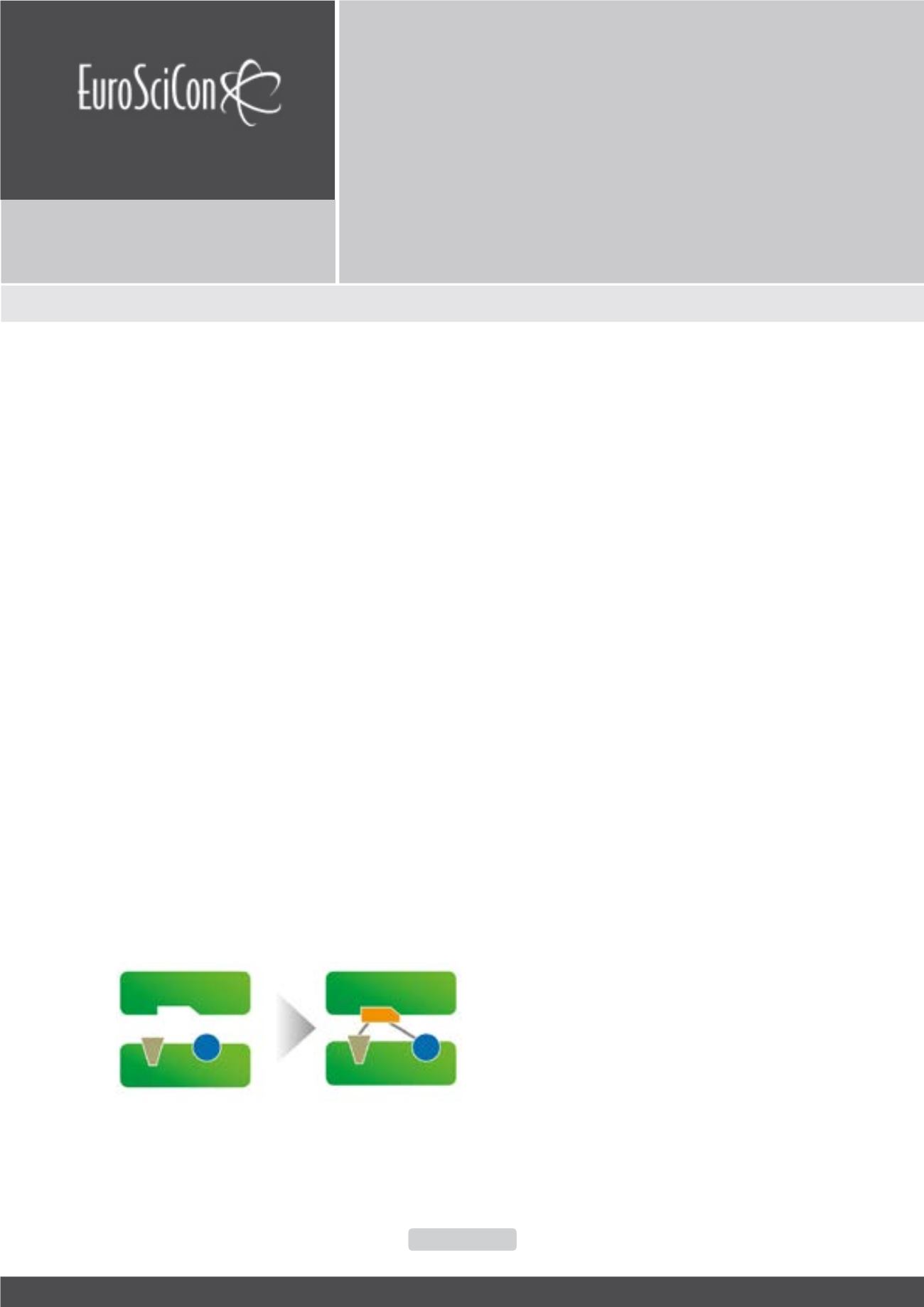
Figure 1:
Fragment-based lead discovery takes advantage of fragment evolu-
tion and linking. Small individual fragments with inherently low affinity but high
efficiency are grown according to the structural model. The efficient binding of
the fragments generates a lead structure in the nanomolar affinity range.
Recent Publications
1. Huschmann F, Linnik J, Sparta K, Ühlein M, Wang X,
Metz A, Schiebel J, Heine A, Klebe G, Weiss M, Mueller
U (2016) Structures of endothiapepsin-fragment
complexes from crystallographic fragment screening
using a novel, diverse and affordable 96-compound
fragment library. Acta Cryst F 72:346-355.
2. Schiebel J, Radeva N, Krimmer S, Wang X, Stieler M,
Ehrmann F, Fu K, Metz A, Huschmann F, Weiss M, Mueller
U, Heine A, Klebe G (2016) Six Biophysical Screening
Methods Miss a Large Proportion of Crystallographic
Discovered Fragment Hits: A Case Study. ACS Chem.
Biol. 11:1693-1701.
3. Schiebel J, Radeva N, Köster H, Metz A, Krotzky T,
Kuhnert M, Diederich W, Heine A, Neumann L, Atmanene
C, Roecklin D, Vivat-Hannah V, Renaud JP, Meinecke
R, Schlinck N, Sitte A, Popp F, Zeeb M, Klebe G (2015)
One Question, Multiple Answers: Biochemical and
Biophysical Screening Methods Retrieve Deviating
Fragment Hit Lists. ChemMedChem 10:1511-1521.
4. Hajduk P, Greer J (2007) A decade of fragment-based
drug design: strategic advances and lessons learned.
Nature Reviews Drug Discovery 6:211-219.
5. Rees D, Congreve M, Murray C, Carr R (2004) Fragment-
based lead discovery. Nature Reviews Drug Discovery
3:660-672.
Biography
Christin studied Biotechnology and joined Jena Bioscience GmbH in 2005. She
was promoted Head of Macromolecular Crystallography & Cryo-EM in 2011 and
works in product development ranging from classic crystallization screens to
specific tools and screens for Cryo-EM
christin.reuter@jenabioscience.comFRAG XTAL SCREEN FOR DIRECT CRYSTALLOGRAPHIC FRAGMENT
SCREENING
Christin Reuter
Jena Bioscience GmbH, Germany
Christin Reuter, Struct Chem Crystallogr Commun 2018, Volume 4
DOI: 10.21767/2470-9905-C1-005














