
Page 47
Journal of Organic & Inorganic Chemistry
ISSN 2472-1123
2
n d
E d i t i o n o f E u r o S c i C o n C o n f e r e n c e o n
Chemistry
F e b r u a r y 1 9 - 2 0 , 2 0 1 9
P r a g u e , C z e c h R e p u b l i c
Chemistry 2019
T
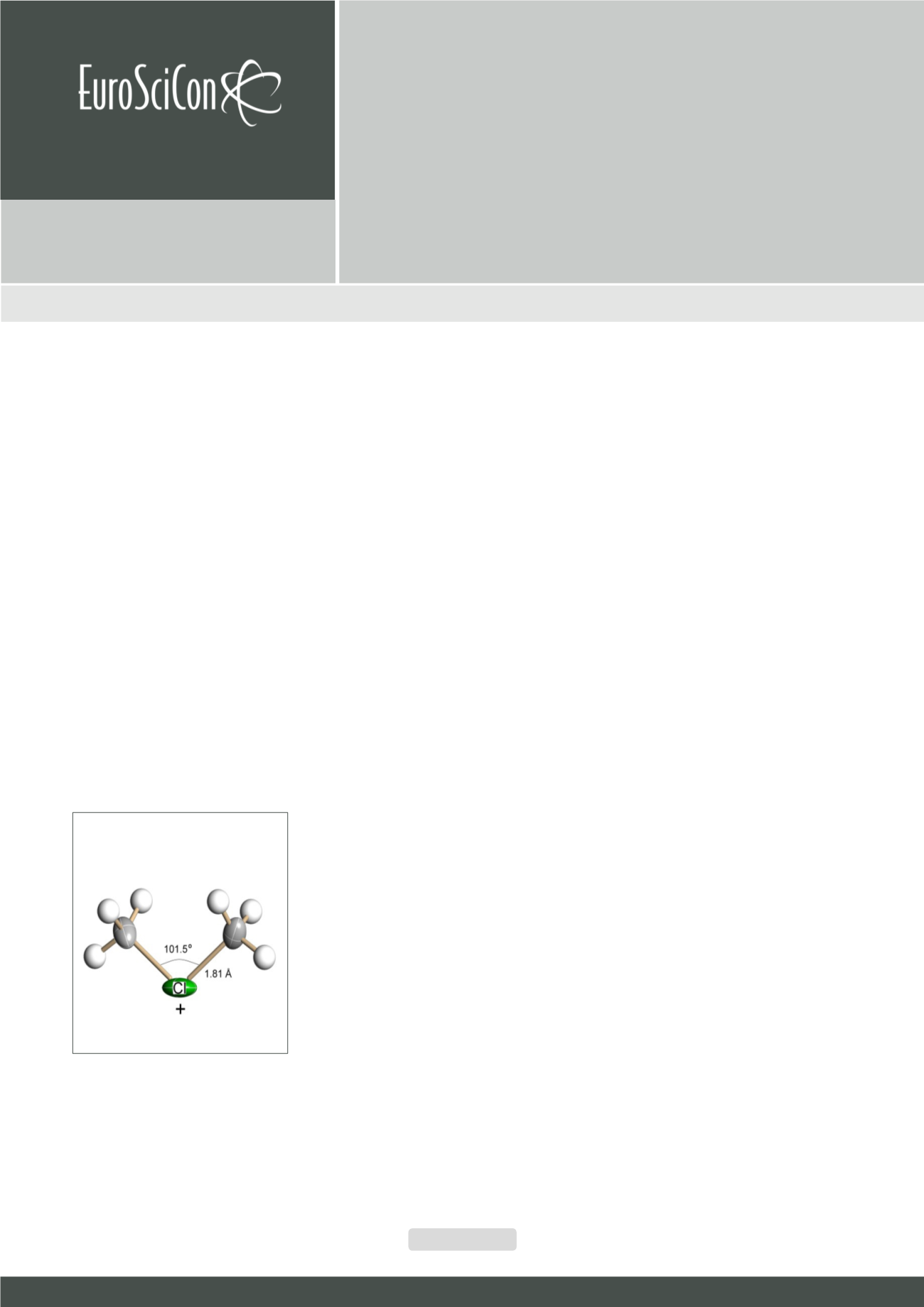
he simple chloronium salts with exceptionally stable carborane anion, R2Cl
+
(CHB
11
Cl
11
¯), with R=CH
3
(see Figure), C
2
H
5
and
(CH
3
)
2
CH are stable at room temperature and serve as intermediates in obtaining different carbocations. Some chemical
properties of chloronium ions in dichloroethane solutions and in the solid salts at room and elevated temperatures (up to 150°C)
are reported. Decomposition of chloronium salts resulted in the formation of the high purity carbocation salts. Their detailed
IR spectra showed a strong discrepancy with the generally accepted theory of hyperconjugative stabilization of saturated
carbocations, based on the modern ab initio calculations. We proposed a refined theory that allowed us to interpret the IR
spectra of all carbocations having sp
2
carbon atom in gaseous, liquid, and solid phases. It was proved that CH
3
/CH
2
groups of
carbocations, which are involved in hyperconjugation, show the stretch and band vibration typical for those of corresponding
groups of the neutral alkanes (that is retain their isoelectronic nature) in IR spectra. Hyperconjugation and polarization are equally
important and closely linked in stabilization of carbocations: the strengthening of one effect weakens the second and vice versa.
We obtained the salts of unsaturated chloronium cations and unsaturated carbocations with conjugated multiple CC bonds,
which are stable at ambient conditions for the first time.
stoyanove48@gmail.comChloronium cations and carbocations in
condensed phases: stabilization and properties
Evgenii S Stoyanov
1,2
1
N N Vorozhtsov Novosibirsk Institute of Organic Chemistry SB RAS, Russia
2
Novosibirsk State University, Russia
J Org Inorg Chem 2019, Volume: 5
DOI: 10.21767/2472-1123-C1-021
















