
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 94
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Two-dimensional inorganic electride promoted electron transfer efficiency in transfer hydrogen reactions
of carbon-carbon multiple bonds
Ye Ji Kim, Byung Il You
and
Sung Wng Kim
Sungkyunkwan University, Republic of Korea
T
he development of simple and efficient chemical transformation routes with maximal yields has been a continuously pursued
challenge in synthetic chemistry. Such protocols can provide important benefits in the field of organic synthesis such as saving
starting materials, reagents, and energy, thereby lowering production costs and environmental impacts. Among fundamental
reactions in synthetic organic chemistry, the reduction of organic functional groups with carbon–carbon (C–C) multiple bonds is
one of the most universally applied and crucial synthetic processes in academic and industrial circles. For efficient electron transfer
hydrogenation, it is essential to use an agent with a high reduction potential to facilitate the electron transfer. In this regard, many
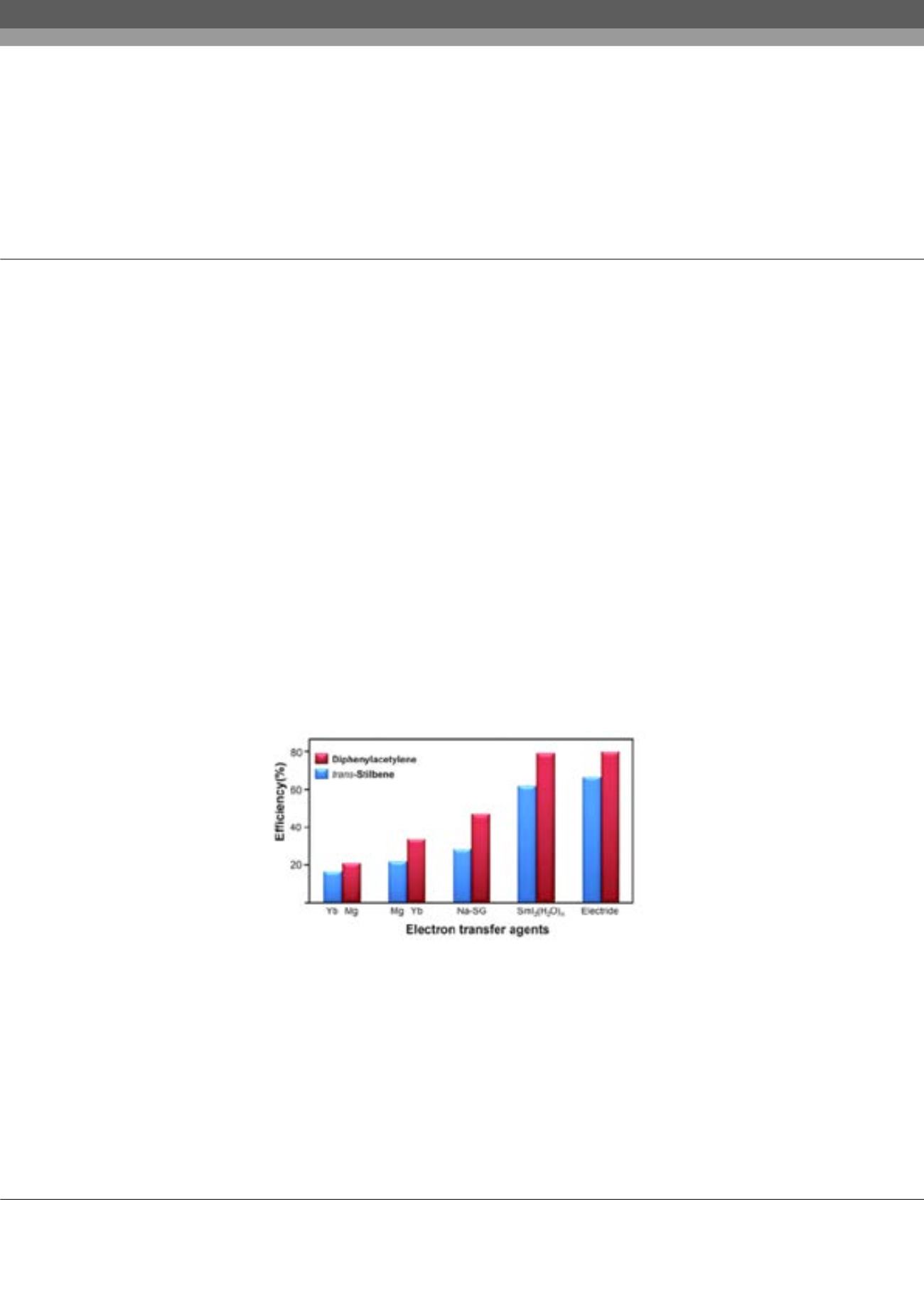
types of materials, such as simple metals (Mg and Yb), stabilized alkali metal systems (Na in silica-gel and in ammonia liquid), and
lanthanide iodides (SmI
2
and TmI
2
), have been employed in electron transfer hydrogenation. Despite their effectiveness in the reported
transfer hydrogenations, there are several drawbacks in the methodology. Major disadvantages include the toxicity, the cost of agents
and the rigorous reaction conditions. Furthermore, the separation of products from the resultants is laborious and inefficient, yielding
pollutants. Of all these drawbacks, the low electron transfer efficiency of the reaction is the most critical issue to be addressed for
efficient transfer hydrogenation. In this presentation, we will introduce simple and highly efficient transfer hydrogenation of alkynes
and alkenes by using a two-dimensional electride, dicalcium nitride ([Ca
2
N]+•e−), as an electron transfer agent. Excellent yields in
the transformation are attributed to the remarkable electron transfer efficiency in the electride-mediated reactions. We found that the
choice of solvent was crucial for enhancing the electron transfer efficiency, and a maximum efficiency of 80% was achieved by using
a DMF mixed isopropanol co-solvent system. This is the highest value reported to date among single electron transfer agents in the
reduction of C–C multiple bonds.
Biography
Ye Ji Kim got her Bachelor’s degree in Chemistry from Kyungsung University in 2013. Since 2013, she joined prof. Sung Wng Kim’s group of Sungkyunkwan
University as Post-Graduate student. Her research interest includes chemical application of inorganic electride, single electron transfer reaction, hydrogen evolution
reaction and nano-particle fabrication.
yejikim@skku.eduYe Ji Kim et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-003
















