
Page 62
Journal of Organic & Inorganic Chemistry
ISSN 2472-1123
2
n d
E d i t i o n o f E u r o S c i C o n C o n f e r e n c e o n
Chemistry
F e b r u a r y 1 9 - 2 0 , 2 0 1 9
P r a g u e , C z e c h R e p u b l i c
Chemistry 2019
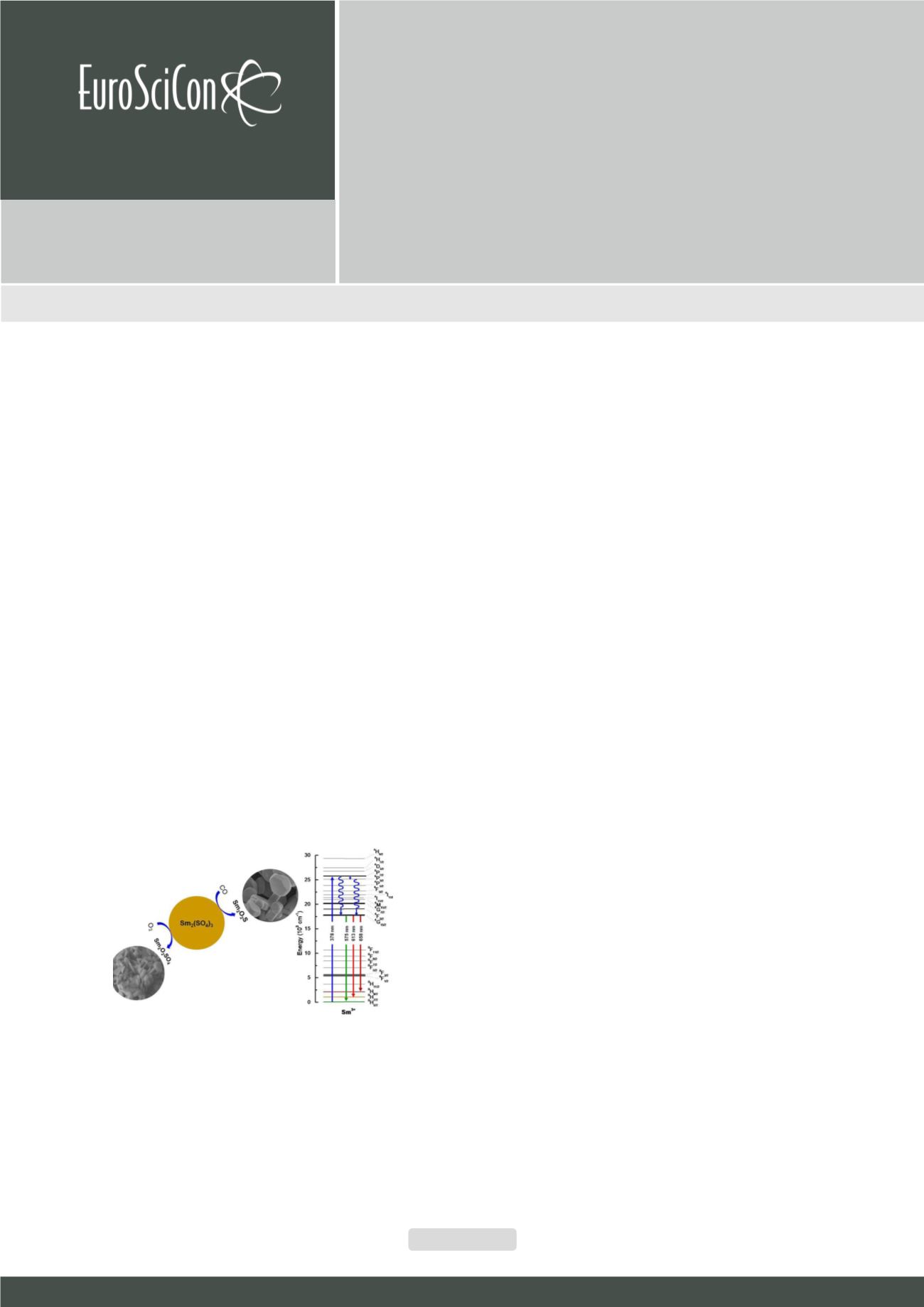
A
spectroscopy study of Sm
3+
phosphors was performed through photoluminescence spectra measurements. The phosphors
were prepared from the thermal decomposition of hydrated samarium sulfate to obtain samarium oxysulfide (Sm
2
O
2
S) and
oxysulfate (Sm
2
O
2
SO
4
). Reddish-orange color emission was monitored from that of oxysulfide phosphor under ultraviolet (UV)
excitation at 6H
5/2
―>4I
13/2
(466 nm) and the photoluminescence emission properties were characterized. The Sm
3+
oxysulfide/
oxysulfate phosphors can be efficiently excited by UV and blue light, and their emission spectrum consists of three important
narrow lines: 4G
5/2
―>6H
5/2
(575 nm), 4G
5/2
―>6H
7/2
(613 nm), and 4G
5/2
―>6H
9/2
(658 nm) intraconfigurational transitions respectively.
The final thermal stability of oxysulfide/oxysulfate phosphors was investigated systematically by TG/DTG measures. Based on
the results, the as prepared Sm
2
O
2
S/Sm
2
O
2
SO
4
materials are promising reddish-orange-emitting phosphors for UV based white
light-emitting diodes.
rodv16429@hotmail.comReddish orange light emitting and thermal
stability of the Sm
2
O
2
S/Sm
2
O
2
SO
4
phosphors
Rodrigo V Rodriguesa
3
, L U Khan
2
, E J B Muri
3
, J R Matos
1
and A A L Marins
3
1
Institute of Chemistry-University of São Paulo, Brazil
2
Brazilian Nanotechnology National Laboratory (LNNano), Brazil
3
Federal University of Espirito Santo, Brazil
J Org Inorg Chem 2019, Volume: 5
DOI: 10.21767/2472-1123-C1-021
















