
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 103
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
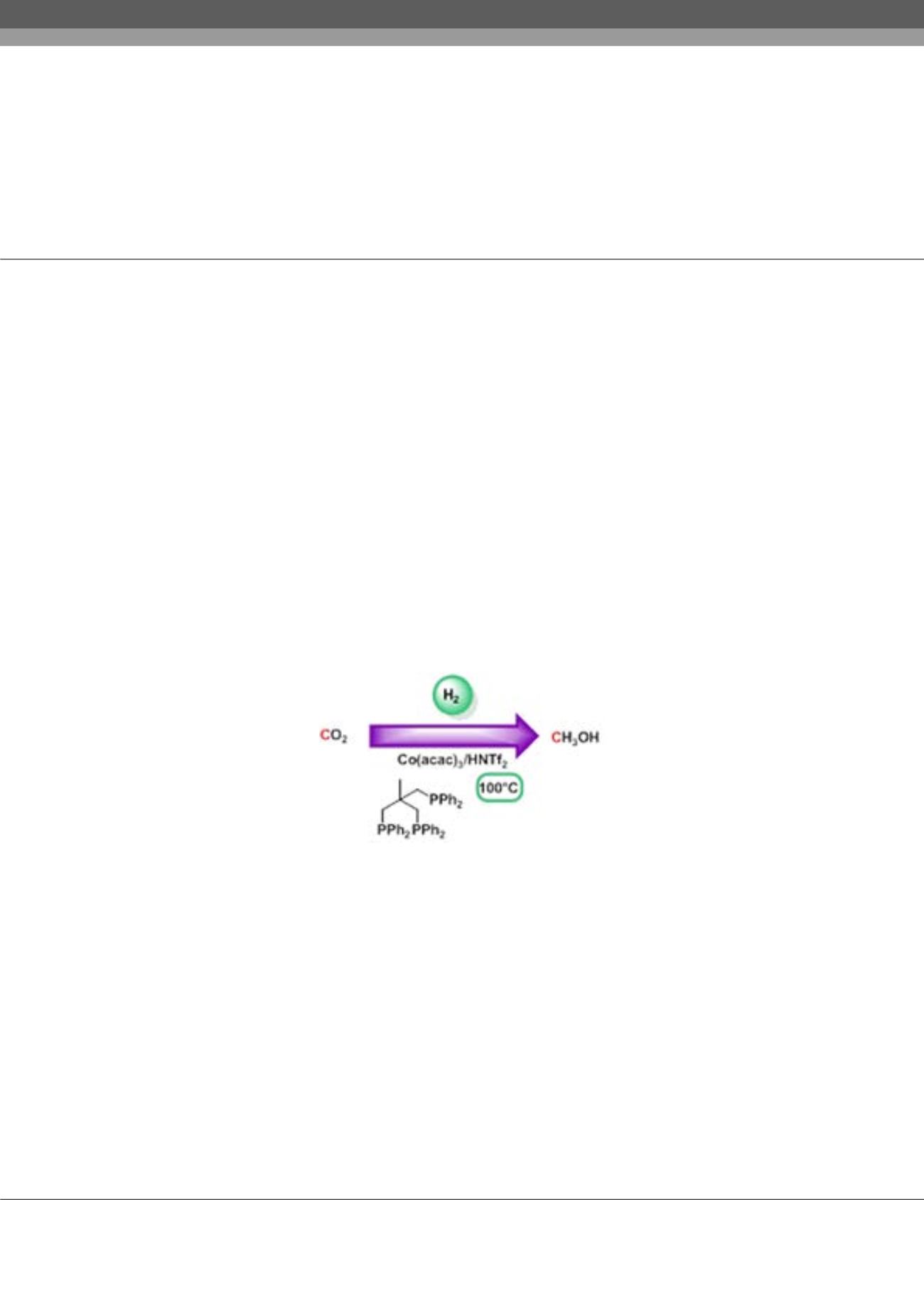
Low-temperature hydrogenation of carbon dioxide to methanol using a homogeneous cobalt
catalyst
Rauf Razzaq
Leibniz-Institute for Catalysis e.V. University of Rostock, Germany
M
ethanol attracts significant attention as a hydrogen storage material (12.5 wt % H
2
), drop-in liquid fuel as well as an
energy carrier in methanol fuel cells. Its industrial utility combined with these promising energy applications has led to
multiple proposals of a so-called “Methanol Economy” in which methanol would be the central carbon and energy feedstock
in a sustainable energy economy. Currently, methanol is produced from fossil fuels, especially natural gas, via syngas (mixture
of CO, CO
2
and H
2
). For a more sustainable production of methanol direct reduction of CO
2
is a highly interesting option
if green hydrogen or renewable energy is used. In such a way it would be possible to recycle atmospheric carbon as part
of a carbon capture and recycling strategy (CCR), avoiding additional CO
2
emissions and replacing non-sustainable carbon
sources. So far, hydrogenation of CO
2
to methanol has been studied intensively using heterogeneous catalysts. Hence, a large
library of active catalysts has been developed but most require high temperatures (>200 °C) to operate. Herein, we describe
the first homogeneous non-noble metal catalyst for the hydrogenation of CO
2
to methanol. The
in-situ
formed catalyst based
on Co(acac)
3
/Triphos/HNTf
2
allows to perform the reaction at 100 °C without a decrease in activity. Kinetic,
in-situ
NMR and
MS studies suggest an inner-sphere mechanism catalyzed by a cationic cobalt/Triphos complex, which is formed after slow
removal of the acac ligands. We hope that this report will inspire the development of novel, homogeneous non-noble metal
based catalysts for a cost and energy efficient hydrogenation of CO
2
to methanol.
Figure: Low-Temperature Hydrogenation of Carbon dioxide to Methanol Using a Homogeneous Cobalt Catalyst
Biography
Rauf Razzaq has his expertise in both Homogeneous and Heterogeneous Catalysis: material design, synthesis and application. Recently, Mr. Razzaq is busy
with designing novel catalytic systems for efficient CO
2
valorization. He has also good experience in chemical reaction engineering and reactor design. During his
research he has not only studied the effect of various metals in catalyzing the CO
2
hydrogenation reaction but has done some extensive work on understanding
the influence of the type of catalytic reactor during such reactions. This approach carries a significant importance in applied catalysis especially in scale-up from
lab to pilot and then industrial scale.
rauf.razzaq@catalysis.deRauf Razzaq, Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-003
















