
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 21
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Lipase-metal integrated catalysis for quantitative conversion of racemic alcohols into optically pure
compounds
Shuji Akai
Osaka University, Japan
H
ydrolase-catalyzed kinetic resolution (KR) of racemic alcohols in organic media is one of the most common approaches used
to obtain optically pure alcohols and their corresponding esters. A wide range of hydrolases, such as lipases and esterases,
have been utilized for KR, many of which are commercially available as solids immobilized on a support and exhibit high catalytic
activity in various organic solvents. Unlike other enzymes, the hydrolases have advantages, such as robustness, high chemo- and
stereo-differentiating ability, applicability to a wide range of non-natural substrates, and lack of cofactor dependency. In addition, the
hydrolase-catalyzed KR of alcohols has distinct benefits in terms of mild reaction conditions as well as the ease and safety of operation
and work-up. However, KR has an inherent limitation as it only gives a maximum 50% yield of products. In this symposium, we
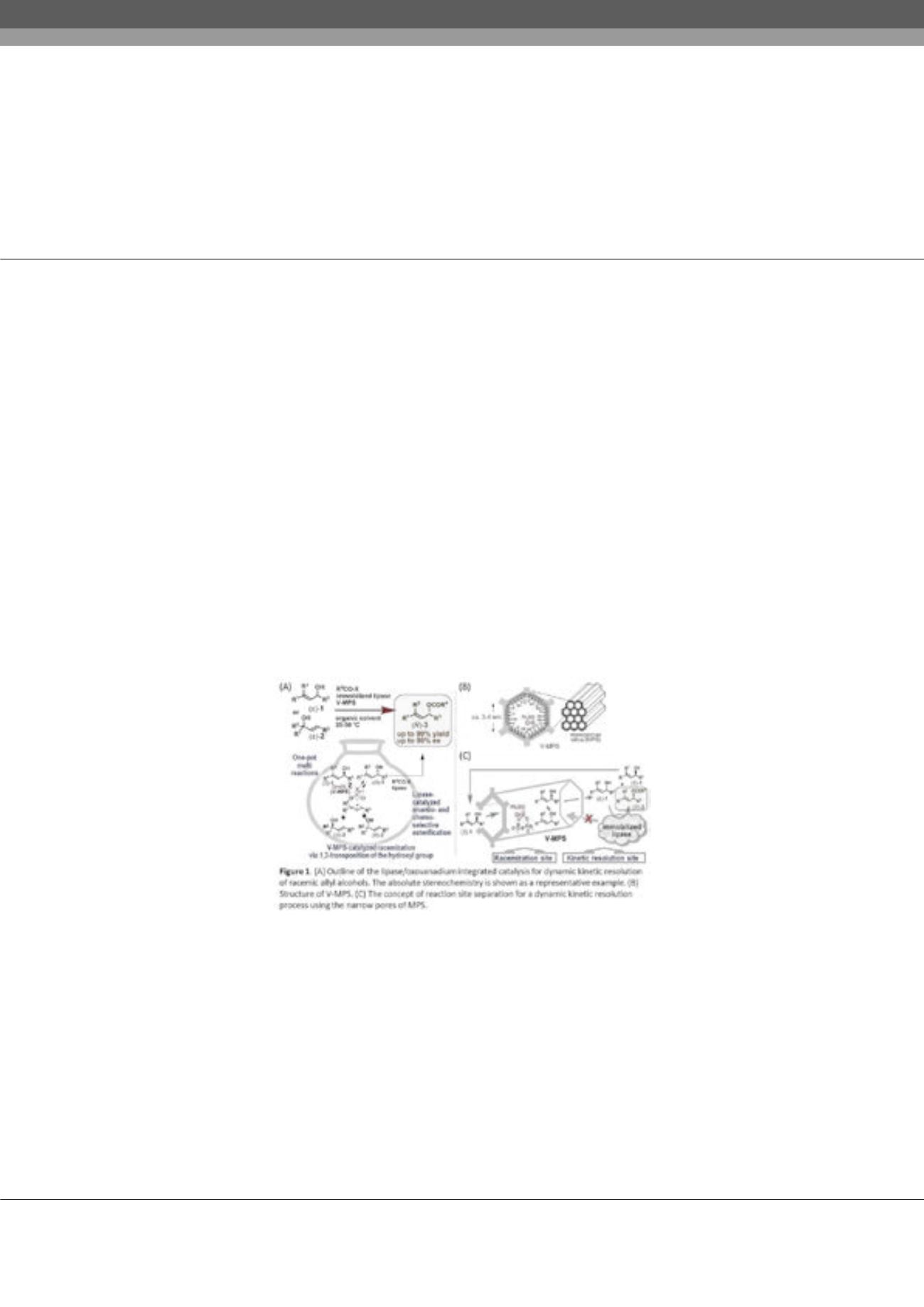
present a dynamic kinetic resolution (DKR) method to overcome this issue. DKR is performed using an integration of the lipase-
catalyzed KR of racemic alcohols and the
in situ
racemization of the remaining less reactive enantiomers by V-MPS, in which the
oxovanadium species is covalently bound to the inner surface of mesoporous silica (MPS) with a pore size of about 3–4 nm (Figure
1B). Its small pore size completely divides the racemization and kinetic resolution sites, and achieves the perfect compatibility
between the oxovanadium moiety and lipases. By the combined use of these two catalysts, racemic alcohols were converted into the
optically active esters (R)-3 with up to 99% isolated yield and 99% ee. The preparation of optically active cycloalkenes bearing all-
carbon quaternary stereogenic centers was developed by direct use of the acyl moiety of the product. The practical application of these
methods has been demonstrated by the asymmetric synthesis of bioactive natural products.
Biography
Shuji Akai obtained PhD degree in 1987 from Osaka University. After two years of Postdoctoral work as a JSPS Research Fellow at Osaka University, he was
appointed as Assistant Professor at Osaka University and promoted to a Full Professor at University of Shizuoka in 2005. Since 2013, he has been a Full Professor
at Osaka University. He was a Visiting Research Fellow with Professor Stephen L Buchwald at MIT during the year 1997–1998. He received the Inoue Research
Award for Young Scientists (1987), the Pharmaceutical Society of Japan Award for Divisional Scientific Promotions (2003), and the Japanese Society for Process
Chemistry Award for Excellence (2005 and 2011). His current research interests include Synthetic Organic Chemistry, Enzymatic Synthesis, Fluorine Chemistry,
and Medicinal Chemistry.
akai@phs.osaka-u.ac.jpShuji Akai, Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-002
















