
Notes:
Volume 3, Issue 2 (Suppl)
Trends in Green chem
ISSN: 2471-9889
Environmental & Green Chemistry 2017
July 24-26, 2017
Page 100
5
th
International Conference on
6
th
International Conference on
July 24-26, 2017 Rome, Italy
Environmental Chemistry and Engineering
Green Chemistry and Technology
&
Dehydration of sugars from grape juice waste by microwave radiation
Almudena Lorente, Covadonga Lucas-Torres
and
Maria Prado Sanchez-Verdu
University of Castilla La Mancha, Spain
I
n Spain, grape industry is the widest, and therefore, the one that generates the larger amount of by-products. The composition
depends on the grape variety used, although that is mainly water (70-80%), and sugars (20%), specifically glucose, fructose and
sucrose, being the rest organic acids such as tartaric, malic or citric. The main reasons for converting those residues are their low pH
and their high BDO (80-89 g/L for white juice, 78-99 g/L for red juice) and high QDO (115-117 g/L for both varieties). We propose
the development of a methodology for the dehydration of the sugars in the grape juice waste water, to obtain 5-hydroxymethylfurfural
(HMF) and levulic acid (LA) as main products. Those chemicals have a great interest as platform compounds, with several applications
in the biofuel industry among others. Preliminary studies were carried out on the pure monosaccharides (glucose and fructose). The
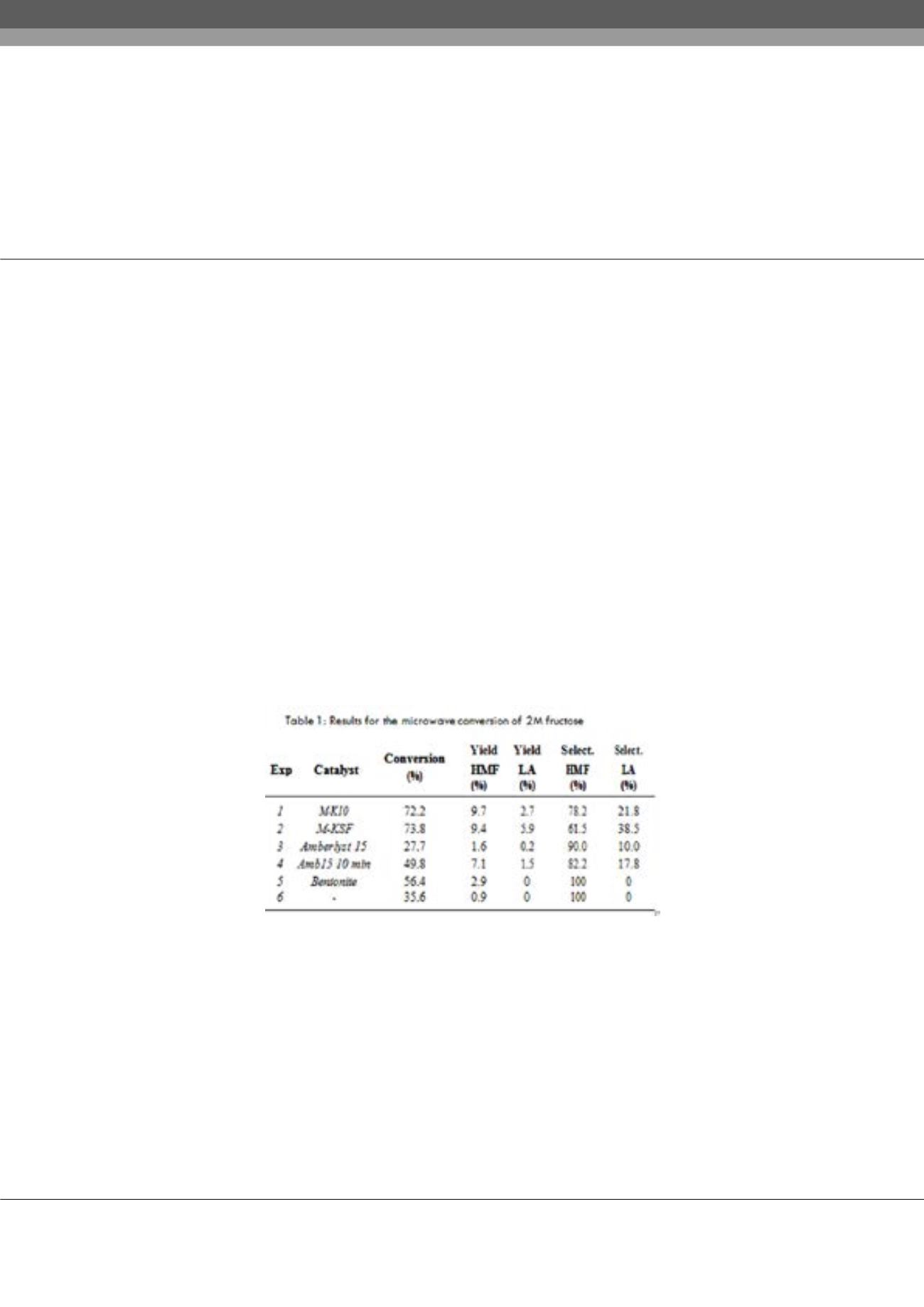
solution (known concentration) was introduced in a microwave vessel and sealed with a cap for under pressure work. The required
heterogeneous catalyst was added (see table 1) and the reaction set at 200°C for 2-15 minutes depending on the sugar. The reaction
crude was dissolved in D
2
O to be analysed and quantified by NMR. The same experiments were carried out, under the optimal
conditions, on several non-edible grape juices, which were provided as a similar residue to waste water sidestreams. The preliminary
studies with fructose and glucose showed that the montmorillonites as catalysts offer the best results. The reactions carried out
with grape juice using montmorillonite KSF were successful in obtaining HMF and LA with a fast dehydration of fructose and a
moderate dehydration of glucose. Also, the catalyst is potentially recyclable which was assessed by several experiments, showing
also a moderate conversion. In this work, we have been able to obtain HMF and LA as biofuel precursors using an alternative energy
source and a potentially recyclable catalyst. Although it shows less efficiency on reusing, it is clean and cheap, and allows us to simply
separate it from the reaction media.
Biography
Almudena Lorente obtained her degree in Chemistry (June 2014) at the University of Castilla La Mancha. Her first contact with Organic Chemistry was in her fifth
year of degree in the group of Organic Green Chemistry and Food and Agro-Industrial Waste Chemistry. Then in November 2014, she started her PhD in the same
group. During these years, she has continued her training in the field of Waste and Bioeconomy. She obtained her certificate in Bioeconomy studies in September
2016.
Almudena.lorente@uclm.esAlmudena Lorente et al., Trends in Green chem, 3:2
DOI: 10.21767/2471-9889-C1-003
















