Research Article - (2018) Volume 8, Issue 5
Nouha Souayed1*, Zohra Haouas2, Ghada Souid3, Abdelfattah Zakhama4 and Naceur A. Boughattas1
1Laboratory of Pharmacology, faculty of Medecine,University of Monastir, 5019 Monatir, Tunisia
2Laboratory of Histology Cytology and Genetics, Faculty of Medicine, 5019Monastir, Tunisia
3Laboratory of Mycotoxines, Phycotoxines and Associated Pathologies, Faculty of Pharmacy, Monastir 5019, Tunisia
4Laboratory of Anatomo-Cyto-Pathologiste, Hopital Fattouma Bourguiba, 5019 Monastir, Tunisia
Corresponding Author:
Nouha Souayed
Laboratory of Pharmacology
faculty of Medecine, University of Monastir
5019 Monatir, Tunisia
Tel: +21656400412
E-mail: souayednouha@yahoo.fr
Received Date: July 30, 2018; Accepted Date: Aug 31, 2018; Published Date: Sep 05, 2018
Citation: Souayed N, Haouas Z, Souid G, Zakhama A, Boughattas NA (2018) Temporal Variation in Murine Kidney Toxicity to the Antituberculosis Agent (Isoniazid). Eur Exp Biol Vol. 8 No. 5:29. doi: 10.21767/2248-9215.100070
Copyright: © 2018 Souayed N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Background: Isoniazid is a drug largely used for both the treatment and prophylaxis of Tuberculosis. In this study, we investigated whether INH-induced nephrotoxicity is influenced by dosing-time.
Materials and Methods: A potentially toxic INH dose (120 mg/kg) was injected by i.p. route to different groups of animals at three different circadian times: 1, 9 and 17 hours after light onset (HALO). INH administration at 1 and 9 HALO resulted in maximum and minimum nephrotoxicity respectively. Toxicity was assessed by the significant increase in both biochemical parameters of kidney function (Urea: URE, Uric Acid: URI and Creatinine: CERT) and stress oxidative (Malondialdehyde: MDA). These results were correlated with the severe and minor renal histopathological observed at 1 and at 9 HALO respectively.
Conclusion: The optimal tolerance or least side effects were detected when INH was injected in the second part of the light-rest span (9 HALO) of mice.
Keywords
Murine kidney; Toxicity; Antituberculosis agent; Isoniazid
Abbreviations
INH: Isoniazid; HALO: Hours After Light Onset; URE: Urea; URI: Uric Acid; CERT: Creatinine; MDA: Malondialdehyde
Introduction
Rhythmicity is a fundamental property of all living organisms. Biological rhythms are regular and periodic incident phenomena in living systems. They can affect sensitivity to drugs, symptoms of various diseases and many other interactions between an organism and its environnement. In rodents and other mammals, rhythms are controlled or regulated by the suprachiasmatic nuclei [1]. Circadian rhythms are physical, mental and behavioral changes that follow a roughly 24 hour cycle, responding primarily to light and darkness of the environment. The role of circadian rhythms has recently been reviewed for the cardiovascular system [2], metabolic syndrome [3], and the gastrointestinal system [4], etc. The role of the kidney in maintaining proper blood pressure rhythm is well known. The renal functions oscillate in a circadian manner with daily fluctuations in renal blood flow, glomerular filtration rate [5] and the excretion of electrolytes such as sodium and potassium [6]. Likewise, urinary excretion of phosphate, magnesium, and acid oscillates with a circadian pattern [7]. Circadian rhythms in both desired and undesired effects of chemical and physical agents are well documented [8-10]. Thus, dosing a medication at the proper biological time with reference to circadian rhythms can result in modulation of its toxicity [11,12] .
The administration of INH is associated with a mild increase of liver enzyme activities in plasma and severe hepatotoxicity in mice [13]. INH can also cause nephrotoxicity [14] which consists of an acute renal failure with tubular necrosis, interstitial nephritis with or without mild glomerular lesions, rapidly progressive glomerulonephritis and light chain proteinuria [15].
The current study is aimed at determining the optimal dosing time inducing least toxicity in order to apply an INH chronotherapy protocol in patients.
Materials and Methods
Animals and synchronization
Male Swiss albino mice aged 8 to 10 weeks were obtained from the Company of Pharmaceutical Industries of Tunisia (SIPHAT, Tunis, Tunisia). They were synchronized for 3 weeks [16] by a regimen of 12 h light alternating with 12h darkness (L/D: 12/12). The light-dark cycle as well as the temperature (23 ± 2°C), humidity (60 ± 10%) were daily controlled. Time series of the rectal temperature were used as a marker of circadian synchronization [17]. Time is expressed as hours after light onset (HALO). All experiments were performed according to the established guidelines for the care and use of laboratory animals.
Drug
INH has an empirical formula of C6H7N3O. It was freshly prepared each day before injecting by adding an adequate volume of sterilized physiological saline. The sublethal dose (120 mg/kg) was administred in mice by i.p. route in a fixed fluid volume (10 ml/kg b.w.).
Study design
The control and treated animals were divided into three groups, each corresponding to the 3 circadian dosing-times (1, 9 and 17 hours after light onset, HALO). The 15 control mice (5 mice per time point, n=15) received a saline injection and the 45 treated mice (15 mice per time point, n=45) were injected INH at a 120 mg/kg dose by i.p. route.
Blood and tissue collections
After 24 hours of INH injection, the mice were anesthetized by inhalation of diethylether, and the blood samples were obtained from a cardiac puncture at single time 24 hours following injection drug. For the biochemical analysis, the blood samples were immediately put in heparin-treated tubes and centrifuged at 10000 rpm for 15 min at 4°C. The kidneys were immediately excised, washed with ice-cold physiological saline solution (0.9%, w/v). The small representative slices were fixed in 10% neutral buffered formalin for routine histopathology. About 1g of the remaining tissues was cut into small pices, homogenized with an Ultra Turrax homogenizer in 3 ml ice-cold appropriate buffer (Tris-buffered saline (TBS), pH=7.4), and centrifuged at 9000 rpm for 15 min at 4°C. The supernatant was collected for the estimation of superoxide dismutase (SOD) activity, catalase (CAT) activity, glutathione (GSH) and malondialdehyde (MDA) concentrations.
Biohemical parameters of kidney function
Plasma levels of creatinine, urea, and uric acid were determined automatically using the Synchron CXq PRO, auto analyser (Farhat Hached University Hospital, Biochemistry laboratory, Sousse, Tunisia). The results were expressed in UI/L. The standard controls were run before each determination of different biochemical parameters within the expected ranges.
Total protein content: The total protein content in the supernatant of renal tissue was measured following the Bradford method at 595 nm using bovine albumin as the standard [18].
Lipid peroxidation products: Lipid peroxidation was assessed by determining the rate of thiobarbituric acid reactive substances (TBARS) production using the method decribed by Buege and Aust [19]. Briefly, 125 ml of supernatant were mixed with 50 ml of Tris-buffered saline (TBS, pH=7.4) and 125 ml of 20% trichloroacetic acid containing 1% butylated hydroxytoluene (BHT), and centrifuged (10 000 rpm, 10 min, 4°C). Then, 200 ml of supernatant were mixed with 40 ml of HCl (0.6 M) and 160 ml of thiobarbituric acid (120 mM) dissolved in Tris and the mixture was heated at 80°C for 10 min. The absorbance was measured at 530 nm and was proportional to the amount of TBARS formed.
Reduced Glutathione: The GSH level was measured colorimetrically by estimating free SH groups, using 5,5- dithiobis-2-nitrobenzoic acid method of Sedlak and Lindsay [20].
• Catalase activity: The CAT activity was measured according to Aebi’s [21] method and hydrogen peroxide (H2O2) disappearance was monitored kinetically at 240 nm for 1 min at 25°C.
• Superoxide dismutase activity: The SOD activity was analysed in the kidneys according to Marklund and Marklund protocol [22]. The enzyme activity was assayed at an absorbance of 325 nm to indicate the inhibition of autooxidation of pyrogallol. One unit of enzyme activity (U) was defined as the amount of enzyme exhibiting 50% inhibition of the autooxidation rate of 0.1 mM pyrogallol in 1 ml solution. The SOD activity was expressed in U/mg protein.
Histopathological study
The kidneys were removed, fixed and embedded in paraffin. Five micrometer-thick sections were cut. All organ sections (n=6, n: number of sections) were processed and stained with hematoxylin & Eosin for light microscopy Leica DM750, provided with a camera Leica ICC50. Each encoded slide was examined by the same histopathologist, unaware of the treatment or the time of drug administration. So, the lesions were blindly graded from 0 (normal) up to 4 or 5. Grades 4 and 5 corresponded respectively to tissular hemorragy and dilatation of the tubule wall.
Statistical analysis
All values were expressed as means ± SD. One- way ANOVA was applied to test the significance of biochemical data of the different groups. The significance is set at p ≤ 0.05. The time series were analyzed by the Cosinor analysis to validate the circadian rhythms. A rhythm is validated when the amplitude (A: the entire peak to trough difference) of the best-fitting cosine function differs from zero with p<0.05 [23]. The rhythm is then characterized by other parameters in addition to A: the mesor (M: the 24 hour rhythm adjusted mean) and the acrophase (? : the peak time of the cosine function). Rhythm detection is statistically significant when A, M, and ? are given with their 95% confidence limits (91).
Results
Synchronisation of mice
In this study, a circadian rhythm in rectal temperature (computed for the combined data of the different circadians groups) was validated by the cosinor analysis on day 1 before treatment (p ≤ 0.001). The acrophase of this 24h rhythm occurred near the mid-half of the dark-activity span (?= 18.2 HALO ± 0.4 h). The characteristics of the 24 h pattern in rectal temperature confirmed the physiological entrainment of mice to the environmental light (L)/dark (D): 12/12 schedule.
Biochemical indicators of kidney function
Creatinine varied significantly according to a physiological circadian rhythm in control mice with an acrophase located in the middle of the dark-activity phase (? = 17.86 HALO ± 1.3 h) (Table 1). INH treatment induced an increase in the 24 hr-mean of CRET in treated mice (68.52 μmol/l) as compared to controls (53.13 μmol/l). CRET increase varied according to INH circadian dosing-time. It was statistically significant in mice treated at 1 HALO (56%) (Figure 1A).
| Parameters | Treatment | Period | Mesor ± SD | Amplitude ± SD | Acrophase (HALO± h) |
|---|---|---|---|---|---|
| CRET | control | 24 | 53.2 ± 9.3 | 10.1 ± 1.2 | 17.86 ± 1.3* |
| Treated | 24 | 70.6 ± 11.3 | 20.8 ± 2.7 | 1.66 ± 0.9* | |
| URE | Control | 24 | 10.1 ± 0.08 | 1.24 ± 0.09 | 15.66 ± 1.9* |
| Treated | 24 | 11.9 ± 1.8 | 1.24 ± 0.05 | 1.8 ± 0.3* | |
| URI | Control | 24 | 222 ± 25 | 16.7 ± 1.5 | 15.33 ± 2.3* |
| Treated | 24 | 292 ± 34 | 23.2 ± 5.6 | 2.53 ± 0.7* | |
| CAT | Control | 24 | 0.1 ± 0.002 | 0.035 ± 0.009 | 20.13 ± 2.1* |
| Treated | 24 | 0.16 ± 0.003 | 0.06 ± 0.001 | 4.8 ± 0.7* | |
| SOD | Control | 24 | 5.4 ± 1.1 | 1.12 ± 0.09 | 14.53 ± 1.3* |
| Treated | 24 | 3.13 ± 0.9 | 1.4 ± 0.02 | 5.93 ± 1.1 * | |
| MDA | Control | 24 | 18.6 ± 2.9 | 1.36 ± 0.2 | 18.8 ± 2.2* |
| Treated | 24 | 19.4 ± 4.2 | 4.47 ± 1.9 | 1.66 ± 0.26* | |
| GSH | Control | 24 | 41.7 ± 11.1 | 4.16 ± 0.8 | 16.4 ± 1.4* |
| Treated | 24 | 65.6 ± 12.5 | 10.01 ± 1.4 | 1.13 ± 0.3* | |
| histological lesions | Control | 24 | No rhythm | ||
| Treated | 24 | 2.51 ± 0.9 | 2.21 ± 0.9 | 0.66 ± 0.1* | |
Table 1: Characteristics of circadian rhythm parameters of biochemical data in controls and INH-treated mice (120 mg/kg, i.p).
The 24 hr mean circulating URE increased significantly from 9.92 mmol/l in control mice to 12.08 mmol/l in treated ones. In controls and treated mice, URE varied significantly according to circadian rhythm (Table 1).
The highest and the lowest mean URE were observed in mice treated at 1 HALO (12.68 mmol/l) and at 9 HALO (11.35 mmol/l) respectively (Figure 1B).
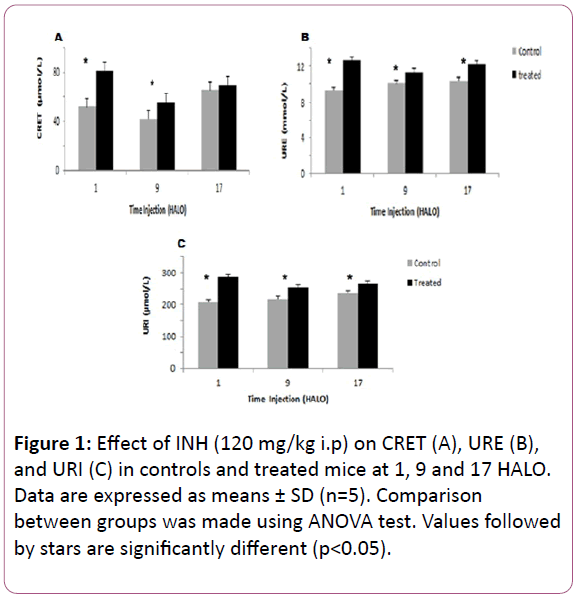
Figure 1: Effect of INH (120 mg/kg i.p) on CRET (A), URE (B), and URI (C) in controls and treated mice at 1, 9 and 17 HALO. Data are expressed as means ± SD (n=5). Comparison between groups was made using ANOVA test. Values followed by stars are significantly different (p<0.05).
INH treatment induced an increase in the 24 hr mean of URI in treated mice (268.16μmol/l) as compared to controls (220.4μmol/l). URI varied significantly in controls and in INHtreated mice (Figure 1C). Similarly, the circadian rhythm in URI appeared reversed between controls and treated mice since the respective acrophases were located at 15.33 HALO ± 2.3 h and 2.53 HALO ± 0.7 h (Table 1).
Oxidative stress in kidney tissue: The highest CAT decrease in the post mitochondrial fraction of the kidney in INH-treated mice was observed at 1 HALO (-60%) (p?0.05) (Figure 2A). In kidney tissue, the circadian rhythm in CAT activity was observed in controls and with an acrophase located at 20.13 HALO ± 2.1h. Such circadian rhythm was modified by INH treatment since the acrophase was shifted to 4.8 HALO ± 0.7 h (Table 1).
The SOD activity in kidney decreased significantly whatever the circadian time of INH injection (p?0.05), but the highest decrease was also observed at 1 HALO (-61%) (Figure 2B). So, the circadian rhythm was observed in INH-treated mice with an acrophase located at 5.93 HALO ± 1.1h (Table 1).
The GSH content in kidney varied significantly according to a physiological circadian rhythm with an acrophase located in the middle of the active phase in control mice (? = 16.4 HALO ± 1.4 h). INH treatment induced a significant increase in GSH activity whatever the circadian dosing time, but the highest increase (51%) was observed at 1 HALO (Figure 2C). In INH-treated animals, the cosinor analysis revealed a circadian rhythm in GSH with the following acrophase: 1.13 HALO ± 0.3 h (Table 1).
INH treatment induced a significant MDA increase in the kidneys (23.7 μmol/mg prot) as compared to controls (19.21 μmol/mg prot) (Figure 2D). A circadian rhythm in MDA levels was statistically validated in control mice with an acrophase located in the middle of the active phase (?= 18.8 HALO ± 2.2h). In treated mice, the MDA level also exhibited a circadian rhythm with an acrophase shifting to the begnning of the light-rest phase (? = 1.66 HALO ± 0.26 h) (Table 1).
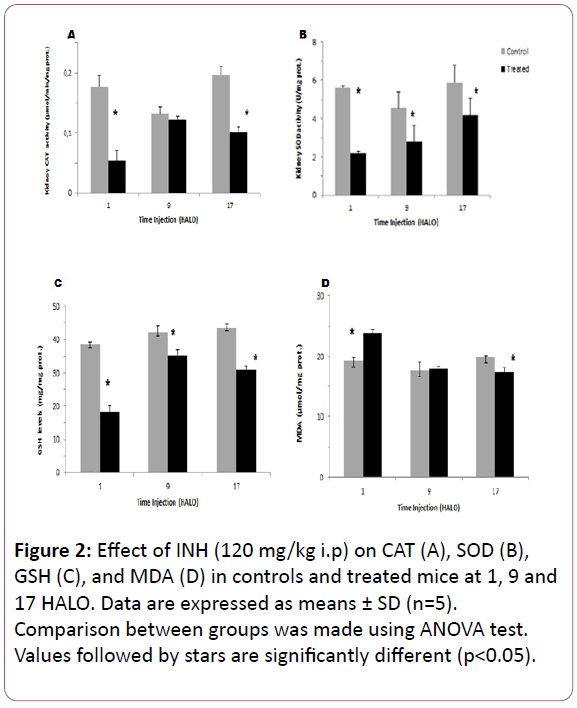
Figure 2: Effect of INH (120 mg/kg i.p) on CAT (A), SOD (B), GSH (C), and MDA (D) in controls and treated mice at 1, 9 and 17 HALO. Data are expressed as means ± SD (n=5). Comparison between groups was made using ANOVA test. Values followed by stars are significantly different (p<0.05).
Histopathological findings: The histological examinations of the kidneys in controls indicated a normal arrangement of cells (Figure 3A). In INH-treated mice, the examination of the kidneys revealed many alterations in both cortical and medulla regions. The kidneys of INH-treated mice at 1 HALO exhibited multiple foci of hemorrhage between the tubules, severe intertitial mononuclear cell infiltrations, and dilatation of the tubules (Figure 3B). However, in mice treated with INH at 9 HALO, there were some mild inflammatory infiltrations in renal tissue (Figure 3C). The kidneys of INH- treated animals at 9 HALO displayed a remarkable improvement in the histological appearance with a marked reduction in induced damage when INH was administered at 1 HALO (Figure 3B,C). As for animals treated at 17 HALO, the kidney damage was less pronounced than that of 1 HALO with a hypertrophy of the glomeruli (Figure D). All of these histopathological changes are graded and summarized (Table 1). Table 2 sums up the circadian rhythm parameters of different biochemical variables (Table 2).
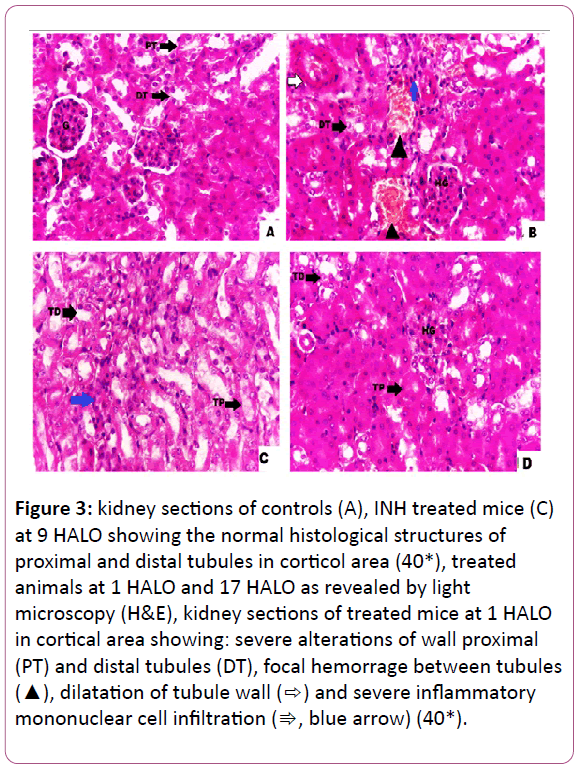
Figure 3: kidney sections of controls (A), INH treated mice (C) at 9 HALO showing the normal histological structures of proximal and distal tubules in corticol area (40*), treated animals at 1 HALO and 17 HALO as revealed by light microscopy (H&E), kidney sections of treated mice at 1 HALO in cortical area showing: severe alterations of wall proximal (PT) and distal tubules (DT), focal hemorrage between tubules (?), dilatation of tubule wall (?) and severe inflammatory mononuclear cell infiltration (?, blue arrow) (40*).
| Treatment | Time of INH administration (HALO) | ||
|---|---|---|---|
| 1 | 9 | 17 | |
| Control | 0.16 ± 0.02* | 0.5 ± 0.01* | 0.33 ± 0.03* |
| INH (120 mg/kg) | 4.66 ± 0.9* | 1.66 ± 0.09* | 2.83 ± 0.1* |
Table 2: Histological score of kidney lesions in controls and INHtreated mice (120 mg/ Kg i.p. at 1, 9 and 17 HALO.
Discussion
As chronobiological studies require animal synchronization with L/D: 12/12 alternation, the rectal temperature was used as a marker of circadian rhythm in mice. The circadian peak of rectal temperature usually occurs in the middle of the dark activity span of mice. This peak location coincides with the occurrence of the highest physical activity in mice during the dark span [13]. Spontaneous rhythms of renal activity have been reported for many years. These diurnal changes are influenced by a 24-hour cycle of activity of hormones engaged in the regulation of renal activity. The dosing of a medication at the proper biological time with reference to circadian rhythm can result in modulation of its efficiency or its toxicity as demonstrated, in particular for the anticancer agents used in human chemotherapy [24].
The administration of antituberculous INH may be associated with a great deal of hematological toxicity at different circadiantimes [13].
The present work aims to investigate whether murine INHinduced renal toxicity varied according to circadian dosing-time.
The data of our investigations showed an alteration of renal function accompanied with the INH administration in terms of significantly and maximally increased plasma concentration of urea, creatinine and uric acid, when INH was injected at 1 HALO.
INH-induced hyperuricemia is in accordance with several previous studies [25]. The elevated urea, uric acid and creatinine concentration indicates malfunction of the kidney due to its damage. It may also indicate that renal blood flow decreased following INH administration [26].
As a measure of renal function status, plasma urea and creatinine are often regarded as reliable markers. It has been reported that plasma concentration of creatinine and urea depends largely on glomerular filtration for their excretion into urine, and the rise of their plasma levels serves as useful indicators of glomerular filtration rate impairement [27]. Thus, the elevation in plasma concentration of these markers in INHtreated mice is indicative of renal injury. Additionally, the elevation of uric acid level was an indicator of vascular disease consisting of thickening of the preglomerular arteries with smooth muscle cell proliferation [28]. The change in these parameters may possibly indicate a reduction in the glomerular filtration rate and a renal dysfunction as a result of INH intoxication associated with an alteration in the renal histoarchitecture. These alterations were manifested as a tubular dilatation, hypertrophy of tubular cells, congestion of renal blood vessels, and interstitial leukocyte infiltration. Our results confirmed the previous findings of Maryam et al. [29] and Nadia et al. [30] who reported that INH induced a histopathological change in mice kidney tissue. It is important to indicate that the leukocyte infiltration in renal tissues of INHtreated mice could be an indicator of glomerular inflammation [31] and that the injection of INH at 1 HALO increased this inflammation. In control animals, the circadian peak of these biochemical parameters of the kidneys occured in the dark activity span of mice. This peak location coincides with the highest activity level, which occurs during the dark span a food consumption of animals [32].
The effects of oxidative stress can be evidenced by cellular accumulation of lipid peroxides. Their reactive metabolites of INH are able to bind to cellular macromolecules [33]. As an alternative to inducing cellular damage by covalent binding, peroxidation of endogenous lipids has been shown to be a major factor in INH cytotoxic action.
Cells possess a complex and precise antioxidant defence system that prevents ROS formation and/or limits their toxic effect. Superoxide dismutase (SOD) is an antioxidant enzyme that catalyses the conversion of the highly reactive superoxide anion radical (O2-) a chain reaction promoter, to less reactive species. This is the first step of systemic antioxidant defense [34]. The antioxidant enzyme catalase (CAT) protects cells from drug-induced oxygen consumption via degradation of hydrogen peroxide or by direct interaction with the drug. Thus, it is essential for cells adaptation to oxidative stress [35]. Under normal physiological conditions, there is an ecological oxidative balance (EOB) between free radical generation and the scavenging capacity of systemic antioxidant defense [36]. In the EOB state, aerobic organisms are maximally protected against the toxic ROS influence [37].
In the kidneys, the maximum decreased SOD and CAT activites was observed in mice treated with INH at 1 HALO, a time which coincides with several previous studies [38].
Several hormones such as melatonin play an important role in generating the circadian rhythm of circulating antioxidant enzymes in mice. So, The peak of the primary antioxidant enzyme of controls was localized at the activity phase.
Melatonin, as a highly electro-reactive molecule, detoxifies electron-deficient reactive oxygen species by electron donation [39]. While melatonin has proven highly efficient in detoxifying the devastatingly toxic hydroxyl radical (OH) and its precursor H2O2, evidence also suggests that it interacts with singlet oxygen (O2), peroxynitrite anion (ONOO-) and nitric oxide (NO) [40,41]. Besides these direct scavenging actions, melatonin increases the activies of several antioxidative enzymes including superoxide dismutase (SOD), catalase (CAT). These antioxidant enzymes (SOD,CAT) limit the effects of oxidant molecules on tissues and are active in the defense against the deleterious effects of oxygen radicals in the cells, which scavenge reactive oxygen species by catalysing the dismutation of superoxide to H2O2 [42].
The present study showed that INH administration induced depletion in the antioxidant activities of CAT and SOD in renal tissues due to the abundant production of O2- and other free radicals in INH-treated mice. Moreover, it has been reported that O2- in addition to singlet oxygen and peroxyl radicals has been shown to directly inhibit the activity of CAT. These observations manifest and explain the significant inhibition of CAT activity in INH-treated mice. Another mechanism that might be involved in the reduced activities of SOD and CAT was through their inactivation by the ROS/RNS production. These findings are in agreement with those reported by other studies [38].
The results of the current study showed that the MDA level increased after INH administration, which is in agreement with previous studies [39]. It was reported that INH metabolism produced by cytochrome P450 leads to the production of reactive oxygen species [40] that induce lipid peroxidation. The elevation of lipid peroxidation is often followed by superoxide dismutase (SOD) overproduction. After this enzyme produced singlet oxygen and hydrogen peroxide, this product is easily converted later into the reactive OH. Both single oxygen and OH radicals have a high potential to initiate the free radical chain reactions of lipid peroxidation. The OH can initiate lipid peroxidation in tissues and MDA is a major oxidation product of peroxidized polyunsaturated fatty acids, and increased MDA content is an important indicator of lipid peroxidation [43]. These results indicate that INH can cause kidney damage. Previous studies revealed a significant increase in malondialdehyde concentrations and reduced activity of antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) in kidney homogenates of mice [44].
Glutathione is one of the most essential compound for maintaining cell integrity [45]. GSH, a non protein thiol, is the primary antioxidant defense system in xenobiotic metabolism and protects tissue from damage. A number of studies revealed that the metabolism of xenobiotics often produced GSH depletion [46]. The decreased concentration of GSH increases the sensitivity of organ to oxidative injury. The depletion of GSH also seems to be a prime factor that permits lipid peroxidation in INH-treated animals [47]. The decreased renal GSH level in INH-treated mice can markedly increase INH toxicity, which was reported by other studies [48,49]. The injection of INH at the beginning of the light-rest phase caused the highest effect of oxidative stress in the kidneys. It is correlated with the maximum histological lesions.
The most important finding of our study is that renal toxicity could be minimized when INH is administred in the second part of the light-rest phase (9 HALO). This time also corresponds to the least hematotoxicity [13].
The overall findings of this study suggest that INH presents a circadian variation in renal toxicity dependent on dosing-time. This must be considered when this medication is administered to patients to manage INH toxic effects [50,51].
In conclusion, it based on the significant time of drug effect, the results of this study might lead to a therapeutic optimization of INH in patients [51-53].