Margaret L Musser1*, Kayden E Toone1, Erika P Berger1, Austin K Viall2, Leslie E Fox1 and Chad M Johannes1
1Veterinary Clinical Sciences, College of Veterinary Medicine, Iowa State University, 1809 South Riverside Drive, Ames, IA, USA
2Veterinary Pathology, College of Veterinary Medicine, Iowa State University, 1809 South Riverside Drive, Ames, IA, USA
*Corresponding Author:
Margaret Musser
Veterinary Clinical Sciences
College of Veterinary Medicine Iowa State University
1809 South Riverside Drive, Ames, IA, USA
Tel: 515-294-4900
E-mail: mmusser@iastate.edu
Received date: June 13, 2018; Accepted date:June 23, 2018; Published date: June 30, 2018
Citation: Musser ML, Toone KE, Berger EP, Viall AK, Fox LE, et al. (2018) Selective Dysmegakaryocytopoiesis Secondary to Chemotherapy in a Dog with Lymphoblastic Lymphoma: A Case Report. J Vet Med Surg. Vol. 2 No. 1:21. doi:10.4172/2574-2868.100021
Copyright: © 2018 Musser ML, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Case History
An 8-year-old female spayed Golden Retriever was presented to the oncology service at the Iowa State University Lloyd Veterinary Medical Teaching Hospital with a history of progressive thrombocytopenia. Twenty-one months prior to presentation the patient was diagnosed with stage IIIa B-cell lymphoblastic lymphoma and received full-course University of Wisconsin-Madison multidrug therapy (Table 1) [1].
| Week |
| Drug |
1 |
2 |
3 |
4 |
6 |
7 |
8 |
9 |
11 |
12 |
13 |
14 |
16 |
17 |
18 |
19 |
| Vincristine 0.5 mg/m2 – 0.7 mg/m2 IV |
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
• |
|
| Cyclophosphamide 200 – 250 mg/m2 PO |
|
• |
|
|
|
• |
|
|
|
• |
|
|
|
• |
|
|
| Doxorubicin 30 mg/m2 IV |
|
|
|
• |
|
|
|
• |
|
|
|
• |
|
|
|
• |
| Prednisone 30 mg/m2 PO q 24 hr |
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prednisone 20 mg/m2 PO q 24 hr |
|
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prednisone 10 mg/m2 PO q 24 hr |
|
|
• |
|
|
|
|
|
|
|
|
|
|
|
|
|
Standard of care substitutions were made as follows: Vinblastine (1.5 mg/m2 IV) for Vincristine on week 6 due to severe lethargy, vomiting, and diarrhea following vincristine treatment. Chlorambucil (1.4 mg/kg PO) for Cyclophosphamide during second round of chemotherapy due to severe neutropenia. Mitoxantrone (4.5 mg/m2 IV) for Doxorubicin during second round of due to the development of dilated cardiomyopathy.
Table 1: University of Wisconsin-Madison canine lymphoma treatment protocol.
A complete clinical remission was achieved for 13 months at which time recurrent lymphadenopathy was noted and the patient was confirmed to be out of remission via lymph node aspirate and cytology. A second full-course of Madison- Wisconsin multidrug therapy was pursued, which resulted in a second complete clinical remission. During the third cycle of the second Madison-Wisconsin protocol, it was determined that the patient was out of remission for a second time based on lymph node palpation and size [2]. Treatment with singleagent mitoxantrone (4.5 mg/m2 IV) was elected. Prior to the administration of the seventh dose of mitoxantrone, the patient was found to be thrombocytopenic with 64,000 plts/μL (RI: 200,000-500,000 plts/μL). Thus, mitoxantrone was delayed.
Upon presentation to the oncology service one week later, the patient was found to be quiet, alert, and afebrile. The peripheral lymph nodes were noted to be stable in size. A complete blood count showed a severe progressive thrombocytopenia (platelet count 20,000 plts/μL; few macroplatelets). An IDEXX 4Dx Plus SNAP was performed to screen for tickborne causes of the thrombocytopenia, which was negative. The patient was also found to be heartworm negative, and was receiving heartworm, flea, and tick preventative medications. The patient had no history of travel outside the Midwest United States. On a CBC completed three days after presentation, the thrombocytopenia was stable (platelet count 32,000 plts/μL; few macro-platelets). A bone marrow sample was taken via sternal aspiration to investigate the thrombocytopenia further. A moderate to highly cellular sample consisting of about 70% nucleated cells and 30-50% lipid vacuoles was obtained. The sample revealed adequate megakaryocyte density and it was noted that proportions of early megakaryocyte precursors were increased relative to normal canine bone marrow. However, substantial morphologic atypia was observed in 90-100% of the cell population including marked anisocytosis, dyssynchronous nuclear to cytoplasmic maturation, and nuclear hypolobulation, all alterations that are consistent with those visualized in dysmegakaryocytopoiesis (Figure 1A-1C). Erythroid hypoplasia was also noted. In an attempt to not exacerbate the thrombocytopenia, subsequent chemotherapy was delayed. Over the following month, the patient’s thrombocytopenia resolved (platelet count reached 214,000 plts/μL; no macro-platelets). The lymph nodes also remained stable in size and thus, additional chemotherapy was not recommended at that time.
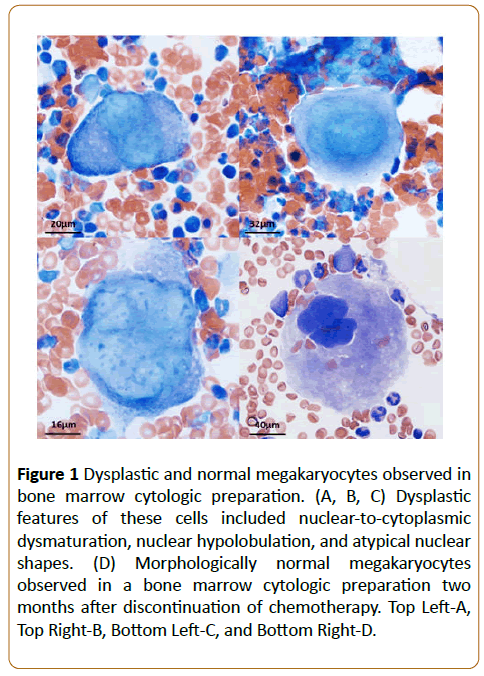
Figure 1: Dysplastic and normal megakaryocytes observed in bone marrow cytologic preparation. (A, B, C) Dysplastic features of these cells included nuclear-to-cytoplasmic dysmaturation, nuclear hypolobulation, and atypical nuclear shapes. (D) Morphologically normal megakaryocytes observed in a bone marrow cytologic preparation two months after discontinuation of chemotherapy. Top Left-A, Top Right-B, Bottom Left-C, and Bottom Right-D..
Two months after the patient’s episode of acute thrombocytopenia, a CBC was completed which revealed a normal platelet count (369,000 plts/μL; no macro-platelets). A second sternal bone marrow aspiration was completed to determine the morphology of the patient’s megakaryocytes and assess the readiness for further chemotherapy. The sample was again adequate with 70% nucleated cells and 30% lipid vacuoles. The samples showed that the megakaryocytes were normal in both number and appearance (Figure 1D). However, due to stable lymph node size, additional chemotherapy was not recommended at that time.
Three months after the episode of acute thrombocytopenia, the patient was noted to have progressive lymph node enlargement indicating loss of remission for a third time and single agent mitoxantrone (4.5 mg/m2 IV) was restarted. This protocol was elected due to concern for the development of doxorubicin-induced cardiomyopathy based on echocardiogram. The patient did not clinically respond to mitoxantrone and developed mild thrombocytopenia (143,000 plts/μL; no macro-platelets). Thus, the prescribed chemotherapy protocol was switched to single agent rabacfosadine (0.8 mg/kg IV). This resulted in complete remission with no noted thrombocytopenia for 2 months, at which time debilitating progressive disease was noted. Due to declining quality of life, the patient was humanely euthanized 27 months after initial lymphoma diagnosis and 6 months after the development and resolution of dysmegakaryocytopoiesis.
Discussion
Myelodysplastic syndromes (MDS) refer to a collection of conditions characterized by the abnormal morphology of one or more of the cell lineages in the bone marrow, and manifest as a non-regenerative anemia and/or pronounced cytopenia of the affected cell lines [3]. Dysmegakaryocytopoiesis, a type of MDS, is defined by the World Health Organization as the dysplasia of ≥ 10% of the total megakaryocytes in a patient’s bone marrow smear [4]. Due to the instrumental role of megakaryocytes in platelet production, this condition typically results in the presence of a severe progressive thrombocytopenia [5]. Morphologic abnormalities seen in the affected megakaryocytes include the presence of multiple, hypolobulated nuclei, granulation of the cytosol, asynchronous nuclear-to-cytoplasm maturation, and a general anisokaryosis of the population [6]. Dysmegakaryocytopoiesis is usually associated with other MDS, most often presenting simultaneously with dyserythrocytopoiesis and dysgranulocytopoiesis. Consequently, reports of dysmegakaryocytopoiesis occurring as a separate entity, medically known as selective dysmegakaryocytopoiesis, are rare in both the human medical and veterinary literature [6].
The MDS, including dysmegkaryocytopoiesis, can be categorized based upon the suspected causative agent [5]. Primary MDS appear to be idiopathic but may be driven by acquired mutations in one or more of the hematopoietic stem cell lineages [5,7,8]. Secondary MDS are associated with toxin exposure, parasitic or viral infection, immune mediated diseases, chemotherapy treatment, and radiation exposure. It does not appear that previous neoplasia itself is a risk factor for the development of secondary MDS [9,10]. A growing body of human literature supports that aggressive chemotherapy or radiation therapy for treatment of a primary neoplasm significantly increases the risk of developing secondary MDS [11,12]. This appears to be especially true in patients receiving alkylating agents, anthracyclines, anthracenediones, and taxanes [7,13,14]. In addition, higher cumulative doses of these compounds substantially increase the risk of secondary MDS development [15].
Secondary dysmegakaryocytopoiesis is triggered by multiple conditions and exposures in dogs, chief among them immunemediated hemolytic anemia and immune-mediated thrombocytopenia [9]. It has also been observed to occur as a result of infection by vector-borne pathogens, notably Leishmania infantum, Anaplasma platys, and Hepatozoon canis, due to the potential for pathological changes in the bone marrow [16]. One case report exists describing 3 dogs developing secondary dysmegakaryocytopoiesis following continuous gamma radiation [17]. However, there remains a paucity of information in the veterinary literature pertaining to dysmegakaryocytopoiesis, and even less pertaining to selective dysmegakaryocytopoiesis. One study sought to characterize and categorize 559 thrombocytopenic canine patients based upon their different clinical syndromes and histories. In this study, selective dysmegakaryocytopoiesis was found to be rare, with only 17 dogs exhibiting the condition. Of those 17, two had been diagnosed with lymphoma, two were diagnosed with leukemia, and one was diagnosed with a mast cell tumor. These five patients were treatment naïve. An additional dog was diagnosed with multiple myeloma and had received melphalan, an alkylating chemotherapy agent [6]. Therefore, there is precedence for the hypothesis that repeated cytotoxic chemotherapeutic protocols and radiation therapy exposure may damage the bone marrow in dogs, leading to myelodysplastic syndromes, including dysmegakaryocytopoiesis.
Over time, MDS itself, or complications secondary to MDS including bone marrow failure due to infectious, hemorrhagic, or anemic stress, can lead to the progression of disease into an acute myeloid leukemia (AML). In some cases, this is a slow progression over a period of years. In other cases, it is a rapid progression that is refractory to treatment and leads to a shorter survival time [18]. Treatment for those with a low to intermediate risk of developing AML focuses on treating the underlying cause (if present) and improving clinical cytopenias via transfusions and the administration of growth factors [19]. Treatment for intermediate to high-risk individuals focuses on slowing disease progression through chemotherapy, demethylating agents, and bone marrow transplantation [18].
Cases of canine MDS are treated similarly to human cases; those with moderate cytopenias can receive transfusions and growth factors, such as epogen [20], whereas more severe cases may require chemotherapy [21]. Cases secondary to a drug treatment, toxin exposure, or treatable disease processes resolve if the inciting cause is removed [10]. Prognosis for canine MDS is varied and depends upon the type (primary or secondary) and successful treatment of underlying causes. Those that progress to AML have survival times less than 5 months [5].
This case report describes the diagnosis and treatment of a dog with suspect secondary selective dysmegakaryocytopoiesis due to exposure to high cumulative amounts of chemotherapy agents. At the time of the patient’s severe, acute thrombocytopenic episode and diagnosis of dysmegakaryocytopoiesis, the patient had received 33 doses of chemotherapeutic drugs using the Madison-Wisconsin protocol for the treatment lymphoblastic lymphoma (Figure 1). Because the thrombocytopenia resolved 6 weeks following the cessation of chemotherapy, and a second bone marrow sample taken two months later showed normal megakaryocyte number and morphology, we believe that the patient’s dysmegakaryocytopoiesis arose largely as a result of the cytotoxic and suppressive effects of the chemotherapy drugs administered upon the bone marrow. In addition, we believe this to represent a secondary selective dysmegakaryocytopoiesis as there was no concurrent evidence of dyserythrocytopoiesis or dysgranulocytopoiesis.
As previously mentioned, there have been multiple publications in the human literature that demonstrate a possible association between the cumulative usages of specific antineoplastic agents in the development of secondary MDS. Most concerning are the alkylating agents, anthracyclines, anthracenediones, and taxanes [13-15]. The Madison- Wisconsin protocol for canine lymphoma includes the administration of cyclophosphamide, an alkylating agent [22], doxorubicin, an anthracycline topoisomerase II inhibitor, and mitoxantrone (as a substitute for doxorubicin), an anthracenedione [23]. Recently, human studies have shown that previous treatment with these agents is a major risk factor for the development of MDS, and a higher risk is present following treatment with mitoxantrone compared to anthracyclines. The effect of cumulative doses of chemotherapy has not yet been definitively elucidated [14]. The vinca alkaloids (vincristine and vinblastine), to our knowledge, have not been associated with the development of MDS.
The mechanism of chemotherapy-induced leukemia and MDS is unknown. However, it likely involves the induction of DNA errors, promotion of these errors during stem cell replication, and subsequent chromosomal aberrations and immunosuppression [24,25]. There is also the hypothesis that those patients with cancer have an underlying predisposition to the development of leukemia and MDS, and this may be exacerbated by chemotherapy indirectly as treated patients live longer, allowing more time for a leukemia or MDS to develop [24].
Whether or not cumulative chemotherapy treatments, or mitoxantrone therapy alone, is implicated in the development of this patient’s secondary selective dysmegakaryocytopoiesis is unclear. However, given the fact that the thrombocytopenia and dysmegakaryocytopoiesis resolved relatively quickly following the cessation of chemotherapy and that there is nothing else in the patient’s full history that has previously been associated with MDS development (including the use of long-term heartworm and flea preventatives), suggests that the condition arose as a result of the cytotoxic effects of the aggressive, cumulative chemotherapy. This is, to our current knowledge, among the first such reports of chemotherapyinduced secondary selective dysmegakaryocytopoiesis in a canine patient.
Conclusion
Although secondary selective dysmegakaryocytopoiesis is a rarely reported condition in both human and veterinary medicine, it nonetheless remains a possible outcome of cancer patients who have been administered repeated rounds of chemotherapeutic drugs. In the case detailed within this report, the suspect secondary selective dysmegakaryocytopoiesis manifested in a severe progressive thrombocytopenia and resolved within months after cessation of chemotherapy. Secondary selective dysmegakaryocytopoiesis may be worth consideration both as a differential and as a contraindication to further therapy with potential causative agents for thrombocytopenic, long-term cancer patients who are receiving high cumulative doses of cytotoxic chemotherapeutic drugs. In addition, cytologic evaluation of bone marrow is a useful clinical tool in investigating thrombocytopenia.
References
- Vail DM, Pinkerton ME, Young KM (2013) Canine lymphoma and lymphoid leukemias. In: Withrow SJ, Vail DM, Page RL (eds.) Withrow and MacEwen’s Small Animal Clinical Oncology. St. Louis, MO: Elsevier, pp, 622.
- Vail DM, Michels GM, Khanna C, Selting KA, London CA (2010) Response evaluation criteria for peripheral nodal lymphoma in dogs (v1.0)- a Veterinary Cooperative Oncology Group (VCOG) consensus document. Vet Comp Oncol 8: 28-37.
- Tefferi A, Vardiman JW (2009) Myelodysplastic syndromes. N Engl J Med 361: 1872-1885.
- Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, et al. (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114: 937-951.
- Weiss DJ, Smith SA (2000) Primary myelodysplastic syndromes of dogs: a report of 12 cases. J Vet Intern Med 14: 491-494.
- Weiss DJ (2004) Selective dysmegakaryopoiesis in thrombocytopenic dogs (1996-2002). Comp Clin Path 13: 24-28.
- Frisch B, Bartl R (1990) Primary and secondary myelodysplastic syndromes. In: Gresham GA, (ed.) Atlas of Bone Marrow Pathology. Hingham, MA: Kluwer Academic Publishers, pp. 67-80.
- Parlier V, van Melle G, Beris P, Schmidt PM, Tobler A, et al. (1994) Hematologic, clinical, and cytogenetic analysis in 109 patients with primary myelodysplastic syndrome. Prognostic significance of morphology and chromosome findings. Cancer Genet Cytogenet 78: 219-231.
- Weiss DJ, Aird B (2001) Cytologic evaluation of primary and secondary myelodysplastic syndromes in the dog. Vet Clin Path 30: 67-75.
- Weiss DJ (2005) Recognition and classification of dysmyelopoiesis in the dog: a review. J Vet Intern Med 19: 147-154.
- Hodgson DC (2015) Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: optimizing treatment for individual patients. Clin Adv Hematol Oncol 13: 103-112.
- Sun LM, Lin CL, Lin MC, Liang JA, Kao CH (2015) Radiotherapy-and chemotherapy-induced myelodysplasia syndrome: a nationwide population-based nested case-control study. Medicine (Baltimore) 94: e737.
- Bhatnagar UB, Singh D, Glazyrin A, Morrmeier J (2016) Paclitaxel induced MDS and AML: A case report and literature review. Case Rep Oncol Med 2016: 1-6.
- Le Deley MC, Suzan F, Cutuli B, Delaloge S, Shamsaldin A, et al. (2007) Anthracyclines, mitoxantrone, radiotherapy, and granulocyte colony-stimulating factor: risk factors for leukemia and myelodysplastic syndrome after breast cancer. J Clin Oncol 25: 292-300.
- Praga C, Bergh J, Bliss J, Bonneterre J, Cesana B, et al. (2005) Risk of acute myeloid leukemia and myelodysplastic syndrome in trials of adjuvant epirubicin for early breast cancer: correlation with doses of epirubicin and cyclophosphamide. J Clin Oncol 23: 4179-4191.
- De Tommasi AS, Otranto D, Furlanello T, Tasca S, Cantacessi C, et al. (2014) Evaluation of blood and bone marrow in selected canine vector-borne diseases. Parasit Vectors 7: 534.
- Tolle DV, Cullen SM, Seed TM, Fritz TE (1983) Circulating micromegakaryocytes preceding leukemia in three dogs exposed to 2.5 R/day gamma radiation. Vet Pathol 20: 111-114.
- Triantafyllidis I, Ciobanu A, Stanca O, Lupu AR (2012) Prognostic factors in myelodysplastic syndromes. Maedica (Buchar) 7: 295-302.
- Mufti GJ, Stevens JR, Oscier DG, Hamblin TJ, Machin D (1985) Myelodysplastic syndromes: a scoring system with prognostic significance. Br J Haematol 59: 425-433.
- Boone LI, Knauer KW, Rapp SW, Stewart JF, Modiano JF (1998) Use of human recombinant erythropoietin and prednisone for treatment of myelodysplastic syndrome with erythroid predominance in a dog. J Am Vet Med Assoc 213: 999-1001.
- Young KM, Vail DM (2013) Canine acute myeloid leukemia, myeloproliferative neoplasms, and myelodysplasia. In: Withrow SJ, Vail DM, Page RL (eds.) Withrow and MacEwen’s Small Animal Clinical Oncology. St. Louis, MO: Elsevier, pp. 662.
- Fleming RA (1997) An overview of cyclophosphamide and ifosfamide pharmacology. Pharmacotherapy 17: 146S-154S.
- Doroshow JH (2011) Topoisomerase II Inhibitors: Anthracyclines. In: Chabner BA, Longo DL, (eds.) Cancer Chemotherapy and Biotherapy: Principles and Practice. Philadelphia, PA: Lippincott William & Wilkins pp. 359, 375.
- Meytes D, Ramot B (1983) Chemotherapy related leukemogenesis. Arch Toxicol Suppl 6: 13-20.
- Pedersen-Bjergaard J, Andersen MT, Andersen MK (2007) Genetic pathways in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Hematology Am Soc Hematol Educ Program 392-397.