Research Article - (2018) Volume 2, Issue 1
Alkhodier H1*, Wang H2, Sun H2, Zhong W2, Cappelli DP2, Liu JA3, Maria-Jose CM3 and Chih-Ko Y3
1Associate Consultant in Pediatric Dentistry, King Faisal Specialist Hospital and Research Center, Riyadh, Saudi Arabia
2Department of Comprehensive Dentistry, School of Dentistry, UTHSCSA, USA
3Department of Developmental Dentistry, School of Dentistry, UTHSCSA, USA
*Corresponding Author:
Haifa A AlKhodier
Associate Consultant in Pediatric Dentistry
Department of Dentistry
King Faisal Specialist Hospital and Research Center
Riyadh, Saudi Arabia
Tel: +966504456094
E-mail: Alkhodier@livemail.uthscsa.edu
Received Date: February 09, 2018; Accepted Date: February 26, 2018; Published Date: March 04, 2018
Citation: Alkhodier H, Wang H, Sun H, Zhong W, Cappelli DP, et al. (2018) Saliva and Oral Health in Attention Deficit Hyperactivity Disorder (ADHD). J Ora Med Vol.2 No.1:2
Purpose: It’s estimated that 9% of children in the United States have been diagnosed with attention deficit hyperactivity disorder (ADHD) and many take medications to control their disease. Since the results of clinical studies on the oral health of ADHD patients have been inconclusive, we compared salivary function and oral health in medicated and non-medicated ADHD and healthy subjects. Methods: Fifty (50) children (6-17 y; female/ male: 1:1.8) were recruited. Group 1 subjects were diagnosed with ADHD and medicated (n=16), Group 2 had ADHD and not medicated (n=17), and Group 3 were healthy (n=17). Whole saliva was collected and flow rate, pH, buffering capacity, and level of secretory Immunoglobulin A (sIgA), cortisol and total protein determined. Nonparametric statistics were used. Results: ADHD children taking medication did not show any significant change in salivary flow rate, pH, buffering capacity, and level of salivary total protein, sIgA, and cortisol compared to non-medicated ADHD and healthy subjects (P>0.5). Streptococcus mutans (S.mutans) has been correlated with caries risk, but in these subjects there was no significant difference between groups. Conclusion: Our results suggest that medicated and non-medicated ADHD patients (6-17 y) have no additional risk for oral diseases or salivary dysfunction compared to healthy controls.
Keywords
ADHD; Saliva; Caries; Streptococcus mutans
Introduction
Attention deficit hyperactivity disorder (ADHD) is a neurodevelopmental disorder in which patients are easily frustrated and characterized by varying levels of inattention, distractibility, impulsive behavior, and age inappropriate activity levels [1]. Typically, ADHD most commonly affects children, with 9% of children in the U.S. having been diagnosed with the disorder, and inordinately affects males over females by an 8:1 ratio [1,2]. About 50% of children diagnosed with ADHD will continue to have ADHD symptoms into adulthood. Brain imaging studies of patients with ADHD show structural and functional abnormalities [3]. ADHD affects academic performance, social interactions, and proper functioning, resulting in a large economic impact on both the patients and their families and society [2]. Management of ADHD includes pharmacological and/or behavioral therapy. The oral health of children with ADHD is a common concern in the dental community due to the side effects of medications used to treat ADHD, improper oral hygiene practices, and sugary food consumption [4,5].
First line pharmacological management of ADHD includes stimulant and non-stimulant medications [2,3]. One of the potential side effects of these medications is dry mouth. Decreased salivary flow rates reduce the buffering capacity of saliva, leading to a lower pH in the oral cavity, which may result in increased numbers of S. mutans and consequently a higher risk of caries [1,6]. To date, reports that describe salivary function in children and adolescents with ADHD are relatively rare and inconclusive. One study showed that children with ADHD, irrespective of medication use or not, had significantly lower salivary flow rates compared to normal controls [7]. In contrast, another study reported no differences in salivary flow rate between ADHD patients and normal controls [8]. Other studies have demonstrated a higher level of caries in children and adolescents with ADHD using the DMFT/dmft index (decayed, missing, filled teeth) and/or caries prevalence [8-10]. However, others have reported no difference in DMFT/dmft index and cariogenic bacteria levels in ADHD patients and controls, although patients with ADHD have a higher plaque index, higher noncavitated caries lesions, and higher caries risk behaviors [1,4,5].
In the current study, we evaluated salivary gland function and oral health in ADHD patients by comparing salivary flow rate, pH, buffering capacity, total protein and secretory immunoglobulin A (sIgA) production, oral Streptococcus mutans levels, and caries risk in medicated and non-medicated ADHD children and healthy controls. In addition, we assessed individual stress levels by measuring salivary cortisol concentrations.
Materials and Methods
Subjects
Children and adolescents (aged 6 to 17 years old) were recruited from the Pediatric Dentistry Clinic and the Ricardo Salinas Dental Clinic, School of Dentistry, at the University Texas Health Science Center at San Antonio (UTHSCSA) between June and December 2016. The study subjects were divided into three groups: Group 1 included subjects diagnosed with ADHD and medicated (N=17), Group 2 subjects were diagnosed with ADHD and non-medicated (N=16), and Group 3 were healthy controls (N=17). Subjects were excluded from the study if they had any of the following conditions: (1) systemic/psychological disease other than ADHD; (2) taking drugs (not for ADHD) known to interfere with salivary flow; (3) undergoing any surgical or chemical procedure affecting salivary secretion. A chart review was conducted to confirm each subject’s diagnosis, medication use, and to assess clinical caries risk using the caries risk assessment tool (CAT), proposed by the American Academy of Pediatric Dentistry (AAPD) and based on three different categories (i.e., biological, clinical and protective factors) [11].
All clinical data/sample collection was performed by one investigator. The drugs used for treating the ADHD group included stimulants (Methylphenidate, Lisdexamphetamine, Dextroamphetamine) and non-stimulants (Atomoxetine [norepinephrine (noradrenaline) reuptake inhibitor (NRI)], Guanfacine, and Catapres [both adrenergic α receptor agonists]). This research study was approved by both Institutional Review Boards (IRB) at the University of Texas Health Science Center at San Antonio and the Audie L. Murphy Division, South Texas Veterans Healthcare System. The IRB approved the proposed study as an expedited protocol due to the very minimal risk posed to the participants. No guardian’s signature was required. However, all guardians were given an IRB approved information sheet at the time of enrollment into the study.
Saliva collection
Subjects were asked to chew on sterilized Parafilm for 1 minute, and then spit whole saliva into a pre-weighed tube for 5 minutes. Collection was divided into a morning session (08:30-12:00) and an afternoon session (12:30-16:30) to facilitate sample collection and increase sample size. Saliva was kept on ice and transferred to the UTHSCSA Sialochemistry Laboratory for processing. Salivary flow rates were calculated from the volume of saliva collected divided by the time elapsed during collection and assuming a specific gravity of 1 [12]. Saliva pH was determined using a microelectrode attached to a pH meter. We measured buffering capacity by adding HCl (5mM) to an aliquot of saliva at a 3:1 ratio [13]. The mixture was gently shaken and the pH measured after 20 minutes [10]. A 100 μl sample of saliva was removed for S. mutans culture and the remaining saliva centrifuged at 1700xg at 4°C for 10 minutes, divided into small aliquots, and then stored at -80°C for future analysis.
Cariogenic microorganisms
Samples of saliva (100 μL) were made up 1 mL with phosphate buffered saline (PBS) and then serially diluted (1:10 and 1:100) with PBS to quantitate the number of S. mutans colony forming units (CFU) present. Mitis salivarius agar plates, containing 0.2 U/ ml bacitracin and 15% sucrose, were inoculated with the diluted saliva samples and then cultured under anaerobic conditions for 48-72 hours at 37°C [14]. At the end of incubation, CFUs were counted and then assigned to 1 of 3 groups based on the number of CFUs/mL saliva present (“high” [≥16,000], “medium” [≥8,000 but <16,000], or “low” [<8,000]). Bacterial counts (CFUs) were then compared to relative caries risk categories (based on the assessment tool approved by the AAPD) using modified cutoff methods.
Determination of total protein, sIgA and cortisol: Total salivary protein was measured spectrophotometrically at 215nm using a DU® spectrophotometer (Beckman Coulter, CA, USA) with bovine serum albumin (BSA) as a standard [15]. Salivary IgA levels were determined using a commercially available Secretory IgA (sIgA) ELISA kit (MyBioSource, San Diego, CA, USA) following the manufacturer’s instructions. Salivary cortisol was assessed using a commercially available Cortisol ELISA kit (Salimetrics®, Carlsbad, CA, USA) following the manufacturer’s instructions.
Statistical Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA, USA). The non-parametric Mann Whitney test was used for two variable comparisons. Kruskal-Wallis test and Pearson Chi-square test were used to compare categorical variables and Tukey's test was used for multiple comparisons. The critical level for statistical significance was P values <0.05, with a confidence interval of 95%.
Results
The demographic information on the study subjects indicated that more males suffer from ADHD than females (Table 1). There was no significant difference in age distribution, gender ratios or time of sample (i.e. saliva) collection between the medicated and non-medicated ADHD and control groups.
Table 1 Demographic information on the study subjects and sample collection time.
| Variable Group | N | Age[Median (25th-75th)] | Male: Female | AM:PM |
|---|---|---|---|---|
| Healthy Controls | 17 | 10 (11-14) | 9:8 | 14:3 |
| Non-Medicated ADHD | 17 | 13.5 (11-15) | 13:4 | 13:4 |
| Medicated ADHD* | 16 | 11 (7.75-12.75) | 10:6 | 11:5 |
*ADHD Medications:Methylphenidate, Lisdexamphetamine, Dextroamphetamine, Atomoxetine, Guanfacine, and Catapres
Median (25th-75th) salivary flow rate for healthy controls was 0.502 (0.349-0.806) ml/min (Figure 1). There were no gender differences in salivary flow rates within the study groups or across all subjects in the population. Salivary flow rates of medicated and non-medicated ADHD patients were not statistically different; in addition, ADHD subjects were not different from their control counterparts.
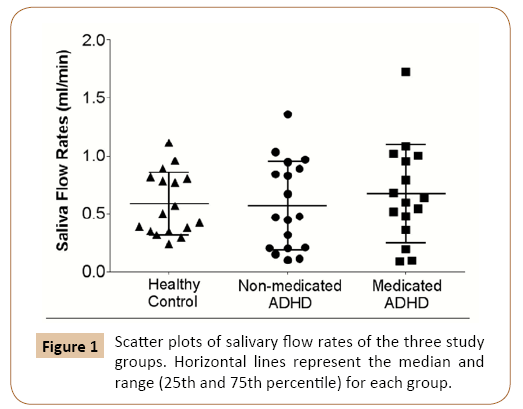
Figure 1: Scatter plots of salivary flow rates of the three study groups. Horizontal lines represent the median and range (25th and 75th percentile) for each group.
Saliva quality was assessed by measuring its pH and buffer capacity, while salivary gland secretory function was evaluated by determining total protein and sIgA levels (Table 2). The results failed to show any significant differences in pH or buffer capacity among the groups (P>0.5). Similarly, total protein and sIgA levels were very similar in all three study groups (P>0.5).
Table 2 Saliva quality and protein secretion.
| Variable Group | sIgA (µg/ml) |
Saliva pH | Buffer Capacity | Total Protein (mg/ml) |
|---|---|---|---|---|
| Healthy Controls | 138.36 (101.41-174.81) |
7.25 (7.14-7.57) |
5.24 (4.18-6.43) |
0.51 (0.24-0.64) |
| Non-Medicated ADHD | 159.35 (73.42-235.79) |
7.25 (7.14-7.57) |
4.83 (4.28-5.55) |
0.45 (0.30-0.54) |
| Medicated ADHD | 106.63 (74.46-223.20) |
7.42 (7.20-7.49) |
5.45 (4.84-6.19) |
0.51 (0.40-0.66) |
Salivary pH, buffering capacity (pH value), total protein and sIgA levels were performed as described in the Methods.
The values shown represent the median and the range (75th-25th). Non-parametric statistics showed that there were no significant differences between the groups.
A caries risk score was assigned to each subject based on the AAPD-endorsed caries assessment tool (CAT) which includes considerations for hypomineralization spots, caries experience, medical need, fluoride use, sugar consumption and socioeconomic status. A majority of the participants in this study were in the high caries risk group and no significant differences were found between the medicated and non-medicated ADHD and control subjects (Table 3). The presence and amount of the cariogenic bacterium, S. mutants, was also assessed. No significant differences S. mutans levels were observed in medicated or nonmedicated ADHD subjects versus healthy controls. Irrespective of study group, S. mutans counts (CFUs/mL saliva) and CAT categories, using the cutoff criteria described, showed a good correlation based on Spearman’s rank correlation, r=0.7792, P<0.001) (Table 3).
Table 3 Caries Risk and Salivary S. mutans Load.
| Variable Group | Caries Risk* | S. mutanscounts# | ||||
|---|---|---|---|---|---|---|
| Low | Medium | High | Low | Medium | High | |
| Healthy Controls | 0 | 4 | 13 | 0 | 4 | 13 |
| Non-Medicated ADHDt | 4 | 0 | 12 | 4 | 0 | 11 |
| Medicated ADHD¥ | 3 | 3 | 10 | 3 | 2 | 10 |
*Caries risk was determined by a caries assessment tool, recommended by the AAPD, which is based on clinical findings, biological and protective factors.
#Salivary S. mutans burden was determined using Mitissalivarius-bacitracin agar (see Methods). S. mutans counts were grouped based on colony forming units (CFUs/mL saliva): ≤8000 (Low); 8000>CFU/ml<16000 (medium) and ≥16000 (High).
t One non-medicated ADHD patient had a different kind of bacterial growth.
¥ One medicated ADHD patient had a different kind of bacterial growth.
Since salivary cortisol levels have been linked with the incidence of caries in children [16,17], salivary cortisol levels were measured and not found to be different among the three study groups (Figure 2).
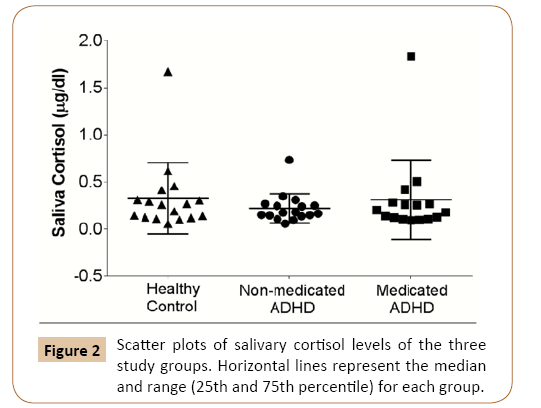
Figure 2: Scatter plots of salivary cortisol levels of the three study groups. Horizontal lines represent the median and range (25th and 75th percentile) for each group.
Discussion
The current study compared salivary function and caries risk in children and adolescents with ADHD taking medications with nonmedicated ADHD subjects and non-ADHD controls. The results indicated that medications had no apparent impact on salivary flow rate, pH, buffer capacity, and secretion of total protein and sIgA. Patients on ADHD medications do not have any additional caries risk as compared to non-medicated ADHD patients and control subjects recruited from public pediatric dental clinics. In addition, ADHD medications had no effect on stress or salivary cortisol levels.
Mouth dryness is a well-documented subjective side effect of ADHD medications [18]. However, whether these medications affect salivary gland function in ADHD children is inconclusive [1]. The current results are consistent with those of Grooms, et al. showing no difference in salivary flow rates between ADHD subjects and non-ADHD controls [8]. Further, others have showed no significant difference in unstimulated whole saliva flow rates (USF) in non-medicated and medicated ADHD children. However, USF in ADHD patients as a group vs. controls was reduced [7]. One possible reason for this discrepancy may be due to differences in saliva collection. In the current study, whole saliva production was stimulated because previous studies were unable to obtain reliable flow rates as the children were unable to chew parafilm and spit simultaneously [12]. Additionally, Hidas et al suggested using an alternative method (other than the unstimulated saliva collection technique) when obtaining saliva samples from ADHD children [7]. Although salivary flow rates are known to be influenced by gender and time of day [19], there was no significant difference in these parameters between the three groups. Whether our current saliva collection technique in ADHD children reflects true daily salivary flow rates will require further validation.
Saliva pH, buffer capacity and protein levels are important for oral health. Our results confirm previous studies showing no significant changes in salivary pH and buffer capacity in ADHD subjects [2,20]. A high salivary total protein level has been linked to increased caries activity in children [13]. Another critical property of saliva is antimicrobial activity. Salivary IgAs play a major role in adaptive immunity in the oral cavity and selectively bind to microorganisms, which inhibits microbial colonization [21,22]. No studies have reported an effect of ADHD or its medications on the level of total protein or sIgA levels. The current study is the first to report salivary total protein and sIgA levels in medicated and non-medicated ADHD children and find no effect in either study subjects. Few studies have investigated the composition of saliva in ADHD children with the purpose of discovering new diagnostic biomarkers. For example, salivary oxidative stress thiol proteins and pseudo-cholinesterase were significantly increased, while magnesium was decreased, in ADHD children [20].
Numerous studies have shown that children with ADHD suffer from higher dmft/DMFT scores, enamel caries, and noncavitated caries [4,8-10,23,24]. Higher dmft/DMFT in ADHD subjects is not always demonstrable because study populations are at mixed stages of dentition [7,9]. A group of researchers has studied ADHD subjects at different ages and found that dmft and DMFT were significantly higher at ages 11 and 17 years, but not at 13 years of age [10,25,26]. Therefore, in the current study, AAPD-CAT was used to assess oral health in the children [11]. Medicated and non-medicated ADHD subjects did not have a higher risk of caries as compared to non-ADHD pediatric dental patients. It is of interest to note that children seeking dental care in school and public (municipal) dental clinics have a high caries risk. Previous studies have shown increased plaque indices in ADHD children with or without medication. However, there were no significant differences in the amount of cariogenic bacteria (S. mutans and Lactobacilli) in saliva [1]. Here, salivary S. mutans was cultured and assigned to three group levels (Table 3). Our results demonstrate that the amount of S. mutans is consistent with caries risk, suggesting these cutoff criteria can be used as a general diagnosis for caries risk. In addition, ADHD children, with or without taking medications, did not show any significant change in salivary S. mutans vs controls.
Cortisol is a product of the Hypothalamus-Pituitary-Adrenal axis (HPA-axis) and is associated with stress levels. Cortisol levels are often affected in people with psychiatric disorders [27].
A systematic review and meta-analysis concluded that nonmedicated ADHD subjects have a lower baseline salivary cortisol level compared to controls, suggesting a dysregulation of the HPA-axis in ADHD patients [28]. ADHD medications, such as methylphenidate, may have a significant effect on salivary cortisol levels [29]. Our study was unable to demonstrate a significant decrease in salivary cortisol levels in children with ADHD. This discrepancy may be due to the fact that we took a “snap shot” of the cortisol level, which is circadian-dependent, and missed any significant changes even though we made an effort to vary the times samples were collected. It is intriguing that one study showed that children with rampant caries had increased cortisol levels [17], another study reported higher salivary cortisol levels associated with fewer caries and gingivitis [16]. Whether cortisol in ADHD is associated with changes in oral health is not known in this study.
A limitation of the current study is its small sample size, due to stringent selection criteria (e.g. excluding ADHD subjects also taking other xerostomic medications such as antihistamines) employed to avoid confounding results. Subjects recruited for the study were convenient cohorts, as they were all recruited from two public clinics. Sample collection times were convenient for the subjects and fit into both school and guardian schedules. However, control subjects were found to be at high risk for caries which raises the question whether the salivary gland function and oral health of these subjects is truly representative of the general population. The current study provides evidence contradicting the common belief that ADHD medications render patients at higher risk of caries because of decreased salivary flow. However, large population-based prospective studies will be needed to confirm these results.
Conclusions
Our results suggest that ADHD patients (aged 6-17 y) in public pediatric dental clinics display no additional risk for oral disease or salivary dysfunction compared to healthy controls.
ADHD medications do not have any measurable effect on salivary gland function.
Large population-based studies in medicated and nonmedicated ADHD patients are still warranted to discover any rare confounding factors that may affect oral disease risk and salivary gland function.
ADHD medications do not affect mucosal immunity or general secretory function.
ADHD does not have any effect on the activity of the HPA axis.
Acknowledgements
We would like to thank the front desk staff and dental assistants at the UTHSCSA Center for Oral Health Care and Research and the Ricardo Salinas Clinic. We also extend our deepest appreciation to the children and parents who agreed to participate in this study. Finally, we would like to thank Dr. David Dean (Professor, Comprehensive Dentistry at UTHSCSA) for his careful reading of the manuscript and helpful comments.
Funding
The research was funded by the University of Texas Health Science Center at San Antonio to fulfill the requirements of the Master’s degree for Dr. Haifa Alkhodier at UTHSCSA.
Compliance with Ethical Standards
This clinical research study was approved as an expedited study by the IRB at the University of Texas Health Science Center at San Antonio and assigned approval number: #HSC20160101H.
Conflict of Interest
The authors have no conflict of interest to declare.