Review Article - (2017) Volume 4, Issue 3
Teena Agrawal* and Priyanka Danai
Banasthali University, Rajasthan, India
Corresponding Author:
Teena Agrawal
Banasthali University, Rajasthan, India
Tel: +91-9680724243
E-mail: tagrawal02@gmail.com
Received date: June 24, 2017; Accepted date: July 03, 2017; Published date: July 07, 2017
Citation: Agrawal T, Danai P. Fossil Early Psilophytian & Lycopodian, Spenopsida Lines of Evolution. Br J Res 2017, 4(3):15. doi: 10.21767/2394-3718.100015
Copyright: © 2017 Agrawal T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Evolution of the plants is the very important aspects of the life on the planet. Since early plant life was typically aquatic in nature. It was the assemblage of the many kind of the aquatic algae’s and the other taxa’s of the aquatic importance. Among them the bryophytes are the plants which were amphibious in nature. However, the pteridophytes are typically land plants having well developed vascular bundles as well as other features of the and adaptations. Pteridophytes have the long fossils history; plants were well developed in the whole Paleozoic era. They were flourished well in the late Devonian and the carboniferous period. In that era one can find the number of the examples of fossils plants which were intimidate in evolution of the many kinds of the organs. Lepidocarpales was the assemblage of the organs like structure which have the pioneer’s symptoms of the evolution of the ovules. That review presents the assemblage of the fossil pteridophytes.
\r\n
Keywords
Fossils pteridophytes; Evolution; Adaptations; Land plants
Introduction
The pteridophytes formed the dominant part of the vegetation in the historic past. It was the middle of the Paleozoic era when these plants group was well flourished in every place. This flora of the pteridophytes was very abundant till early Mesozoic but late Mesozoic era was well dominated by the gymnosperms. In modern day the pteridophytes flora is replaced by the spermatophytes. These spermatophytes involved the gymnosperms and the angiosperms. Present era is the best flourished by the angiosperms [1,2].
Ages of angiosperms
In whole world the pteridophytes are now of the relict in distribution. They are restricted to the some of the tropical rain forest and the northern hemisphere of the world. However in India the pteridophytes are distributed in to the Himalayas and the Nilgiris. Here large very beautiful tree ferns can be seen with good physiognomy, similarly epiphytic ferns and the other hanging club mosses can be seen in the Nilgiris hills. In India 500 species of the ferns can be seen with different kinds of the pattern of the foliage, which are the taxonomic significance in nature [3-6].
Pteridophytes plants have the long fossil’s history. They have been recognized in the late Silurian period of the Paleozoic era. These plants have the dominant vegetation in whole of the Paleozoic era. The middle and the late Paleozoic era can be regarded as the age of ferns or ages of pteridophytes. The giant lycopsida and the horse tails and the arborescent tree ferns dominated the whole biota at that time. The pteridophytes which are presented by the lycopsida and sphenopsida and the pteridopsida which are of the length of the maximum 6 to 7 feet’s were very abundant in distribution and in height of the trees [7]. They were reportedly around 120 feet’s at the time of the Paleozoic era. They were very abundant and highly s in whole vegetation. This era was the evolution of the pteridophytes and the evolution of the gymnosperms. However in today world they are represented only by the some relict genera and the relict fossils evidences [8].
That era was dominated by the Lepidodendron, sigilalria and the calamites and other fossil lycopsida of that era.
However distribution of the ferns was the matter of the slightly ambiguity, since ferns were of less diversity in the Paleozoic era but as the evolution proceeds and the time passes the diversity of the ferns increase, well a number of the ferns can be seen in that era with great diversity [9].
In India, the fossil pteridophytes are studied by Surange in detail. He has given the whole account of the places in India, which have the long distribution of the fossils pteridophytes. Among the four classes of the pteridophytes like Psilopsida, lycopsida and sphenopsida and pteridopsida, he has described one of the members of psilophyta, seven fossil members of the lycopodiophyta, 12 members of the sphenophyta and 66 members of the pteriphyta. A large number of the fossil ferns have been described in the Rajmahal hills of the India [10]. Bose, Sah and Sharma have described a number of the fossil ferns from the Bihar and other hills of the India. Suthar & Sharma reconstruct the whole solenopteris from the jurrasic flora of the Rajmahal hills.
They found the plant in the form of the leaves, stems, flower, and the seeds in different forms. These plants organs have been termed with different terminology [11-15].
In India the Gondwana land was the point of distribution of the many fossil ferns & horse tails. Glossopteris flora of the Mesozoic was constituted by the many different genera of the sphenophytaof the pteridophyta.
Some the detailed account of the fossils pteridophyta is described below:
Fossil psilophtales: This was the new class which has the assemblages of the fossil plants. This class was coined in the era of the 1917. In that class few genera was included like the Rhynia, horneophyton and psilophytlaes. These plants were distributed during the late Silurian and the Devonian and the Upper Carboniferous period. These plants lack true roots, leaves and the other structures [16].
However developed vascular system can be seen in these groups. Psilophytles are totally different from the other similarly named class termed as the psilotales (Figure 1) [17-20].

Figure 1: Fossil ferns.
The plant was described by the Kingston and Lang in the rhynia chart of the British islands. Rhynia chert is the place which was formed due to volcanic eruptions. This is the place where a long belt of fossil plants have been observed. The plant was found in different stem, leaves and the isolated sporangia [21]. Typical dichotomous division can be seen in the stems of the Rhynia plants. Horneophyton, is the fossil pteridophytes and it is the linkage between the fossil psilotales and the other living members’ of the spehopsida (Figure 2).
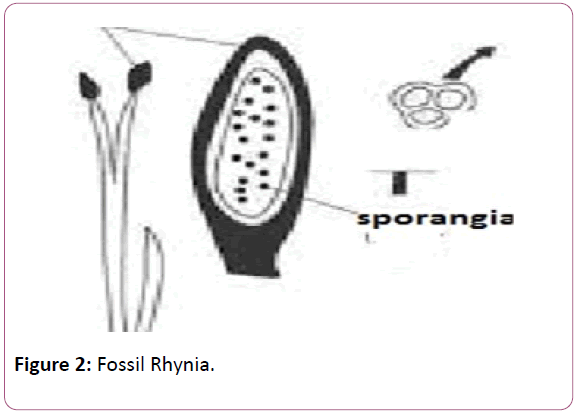
Figure 2: Fossil Rhynia.
These members were also reported form the Rhynia chart of the Scotland in 1920. The sporophytes body is dichotomous in division and the sporangium having the central columella and the other organs of the sores in sides [22].
The presence of the columella in the Hornea is the primitive features of the bryophytes whereas the presence of the tracheids in the sporangia is the presence and the development of the vascular tissue shows the resemblance with the higher plants (Figure 3).
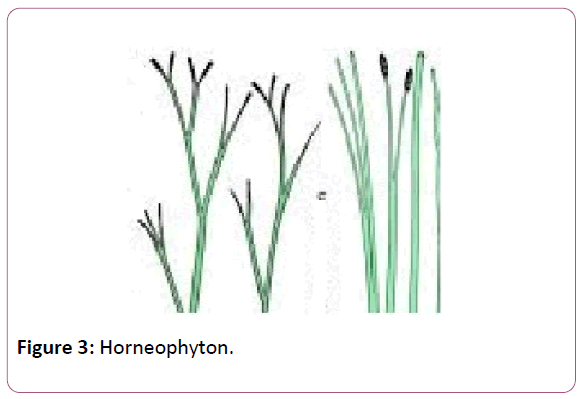
Figure 3: Horneophyton.
Rhynia and horniophyton has been reported form the early Devonian period of the Rhynia Chert of the Scotland. These were the giant and the wet ecosystem of that era.
Fossil lycopsida: The class lycopodiales has the assemblage of the living and the fossil genera’s. This is one of the oldest lineages of the fossil genera’s and the branches [23]. In these fossil genera’s typical heterospory and the alternation of the generation can be seen with clear examples. Some of the fossil genera’s are enlisted in these orders as:
• Lepidodendrales
• Isoetales
• Zosterophyllaeles
Lepidodendrales has the long fossil history, with primitive vascular and arbores cent plants related to the lycopsida [24-26]. This plant group were well flourished in the late carboniferous period, however rapid declines of the lepidodendrales can be seen during the Mesozoic era. These plants group reached to the height up to the 30-40 meters during the devonian and the late Silurian periods (Figure 4).

Figure 4: Reconstruction of the lepidodendrales world.
Lepidodendrales has the long trunk, which reached the height up to the some 40 meters, it was unbranched as well as it bifurcates at the top of the branches [17,20,27]. At the top of the branches one can see the crown of the braches which differentiate it from the rest of the group. Some of the genera’s of the lepidodendrlaes are enlisted as:
• Lepidodendron
• Lepiodpholeois
• Sigillaria
Lepidodendron reproductive structures has-been found in the form of the cones or the separated sporangia [28]. These have been termed as the Lepidostrobus. These cones has always been found in the form of the cones and isolated, not in the form of the attached part of the lepidodendron stem (Figure 5).
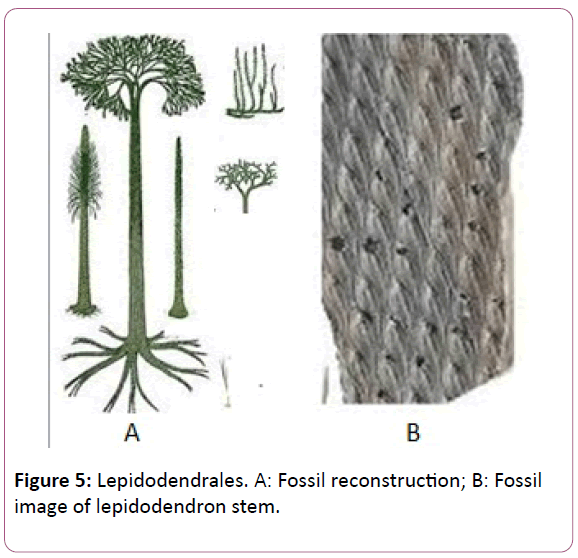
Figure 5: Lepidodendrales. A: Fossil reconstruction; B: Fossil image of lepidodendron stem.
Fossils spenopsida: Spenopsida is the class of the assemblage of the fossil and the living pteidophytes, going back to the late Devonian period, these plants commonly known as the horse tails. Living horse tail represent the genus Equisetum [29,30], which are represented by the 20species, these species grows in to the humid areas and generally in to the groups. The vertical stem is represented by the crowns of the leaves, which are present in the alternate sequences of the stem having the ridges’ and the furrows.
Calamitales are the fossils pteridophytes which were arborescent in the Devonian period, whole and the giant forest of the calamitales can be seen in that era. They were the dominant vegetation of the understory of the coal swamps of the carboniferous period [31-36].
A number of the organ taxa have been identified; whole plants are not reported so frequently. Calamites is the name of the fossil stem, other fossil organ genres are enlisted as:
• Arthropitys
• Astromylon
• Annularia
Annularia is the name of the leaves of the calamites stems. They were arranged in the form of the whorls in the group of the 8-11. They were also reported in the form of the separated organs never found attached in anybody [37-39]. Several fragments of the rocks containing prints of fern like foliage. Pteriopsida were found in the non-technical private collection of rocks, and minerals of costarica. The rocks were collected in the rio general valley by personal of aluminium of America. The matrix proved to be the dark grey luciteberlonging to the terraba formations. After the review of the literatures the ferns like organism were found to be the pteropsida (Figure 6).

Figure 6: Fossils leaves of Annularia spehnopsida.
Conclusion
This review article is the assemblages of the some of the discovered early pteridophytes. Most of the fossil pteridophytes have been reported in the Rhynia Chert of Scotland. These are the linkage of the evolution of the early land plants. Since before Pteridophytes the evolution on the land was a basic and significant step, since these pants have a number of the features of the intermediate evolution. Among them fossil psilophytlaes and the lycopodiales, spenopsides presents the lines of evolution of the further spermatophytes of the gymnosperms and angiosperms.