Keywords
Equine hoof horn quality; Fatty acid pattern; Stratum corneum
Introduction
The quality of hoof horn (HQ) contributes significantly to the use of the horse in sports and consequently also influences the market value of the animal. Currently, no data about the relationship between the composition and quality of the equine hoof horn exist. However, feeding, keeping conditions (e.g., hygiene of barn/ meadow) and soil moisture could be shown as exogenous influences on HQ [1-6] whereas genetic disposition is proved as endogenous factor [7-10]. The intercellular kit of Stratum corneum consists mainly of lipids and free fatty acids [11]. Caprylic acid, capric acid, lauric acid, myristic acid, palmitic acid, stearic acid, oleic acid and linoleic acid were identified as predominant fatty acids in Stratum corneum of dermal epidermis, claw and hoof horn so far [11-13]. In this study we found additional fatty acids, e.g., undecylic acid, palmitoleic acid, margaric acid and heneicosanoic acid that play a role in HQ and hoof horn moisture (HM). Lipids and free fatty acids are responsible for elasticity, water absorption and the microbial barrier of the hoof capsule [1,5,14,15]. Consequently hoof horn of low quality could be a reason for chronic lameness caused by infection or mechanical overstrain of distal joints.
In contrast to the horse, correlations between the fatty acid composition of the claw horn and a lameness disposition for cattle have already been described [11]. The aim of the present study was to establish a link between the composition of fatty acids in the equine hoof horn and its qualitative characteristics.
Methods and Materials
Subjects
For this study, in total 24 horses from a commercial equestrian facility in Goettingen (Germany) were investigated. This includes 17 Hanoverians, 1 Hessian, 1 Oldenburger, 1 Westphalian, 1 Swabian, 1 Trakehner Horse and 1 German Riding Pony. The sampling was carried out randomly from a herd of 90 animals. For this study animals whose hoofs were treated by the chosen farrier were selected. They were all dressage horses (Warmblood). The age of the horses participating in this study ranged from three to 17 years. The gender distribution was 18 geldings and six mares. Environmental factors such as handling, keeping, care and feeding of the horses were standardized. The animals were kept in boxes covered in wheat straw and taken daily for several hours to the local pastures. The feeding consisted of in-house hay, oats and mineral feed by Derby® including Calcium, Phosphor, Magnesium and Natrium, but no minerals that are proven to influence HQ. All horses got the same quality and quantity of mineral feed (15 g/100 kg of body weight). They were ridden 1-2 x daily on a sandy bottom for 45-60 min, dressage level A to L. At the time of sampling, all horses have been wearing horseshoes for at least 12 months with a farrier interval of 8 weeks.
Evaluation of horn quality and moisture
The hoof horn quality was evaluated by a professional farrier. A mark from a scale of 1 to 5 was given in Table 1 with 1=excellent quality, 2=good quality, 3=average quality, 4=poor quality and 5=insufficient quality depending on strength/fragility as well as particular structures like horn cracks. The moisture of the hoof horn has been assessed and divided into the categories moist and dry hoof horn and such with balanced moisture (Table 2).
| |
1=Excellent |
2=Good |
3=Average |
4=Poor |
5=Insufficient |
| Smooth, elastic and regular horn |
✓+ |
✓ |
-- |
-- |
-- |
| Deep cracks in horn wall |
-- |
-- |
-- |
✓ |
✓ + |
| Cracks in surface of horn wall (brittle wall) |
-- |
✓ |
✓ |
+ |
✓ + |
| Break offs in nail holes |
-- |
-- |
-- |
✓ |
✓ + |
| Flaky sole |
-- |
-- |
✓ |
✓+ |
✓ + |
| Irregular structured white line |
-- |
✓ |
✓ |
✓ + |
✓ + |
| Brittle sole (like powder) |
-- |
-- |
-- |
✓ |
✓ + |
| Hollow horn wall |
-- |
-- |
-- |
-- |
✓ |
| Greasy horn |
-- |
-- |
✓ |
✓ |
✓ |
| Black lesions in sole and white line |
-- |
-- |
-- |
-- |
✓ |
✓ =Sometimes/few/small ones
✓ +=always/lots of/great ones
Table 1: Categorization of hoof horn quality.
| |
X=Moist |
Y=Dry |
Z=Equable |
| Horn wall |
greasy |
rough, inflexible |
smooth, elastic |
| Horn sole |
(rough), greasy |
rough, like powder |
smooth, regular, elastic |
| White line |
greasy, irregular, wide |
narrow |
regular |
Table 2: Categorization of hoof horn moisture.
Preparation of samples
The wall and bottom horn samples were taken once per subject by a professional farrier. Samples of all four hooves were pooled per animal. Contaminants were removed thoroughly with tooth brushes (sand, smaller fragments) and tweezers (straw, hay, larger fragments). The horn samples were immediately stored at -20 °C. The frozen horn samples were cut into pieces of about 5 mm3, using hoof cutting pliers. In a laboratory mill by Retsch GmbH, the horn was grounded to powder with a grain size of 0.5 mm. Powder was air dried for 48 h under a laboratory hood, For fat extraction about 30 g have been weighed out.
Fatty acid extraction
The fat extraction was carried out for seven hours with an apparatus according to Soxhlet in petroleum benzine (boiling range 40-60°C. The yield of lipid of about 30 g hoof horn ranged between 0.5% and 1% fat. To determine fatty acid profile about 250 mg extracted fat was necessary. The fat samples were saponified with methanolic potassium hydroxide solution. The soap solution was washed twice with petroleum benzine. Quantitation of fatty acids was done after preparation of fatty acid methyl esters (FAME) with Boron trifluoride-methanol complex (20% solution in methanol).
Gas chromatographic analysis of fatty acid methyl esters
A Shimadzu GC-17A, equipped with a flame ionisation detector and a 50 m BP 21 capillary column of 0.25 mm i.d. and a coating thickness of 0.25 μm was used to separate FAME. Nitrogen was used as the carrier-gas, with a split ratio of 50:1 and 0.8 mL/min column flow rate. The injector temperature was maintained at 250°C. A temperature program with a total run time of 55 min was used. The column temperature, after an initial isothermal period of 5 min at 140°C, was increased to 240°C in increments of 4°C/min, and was maintained at this temperature for 25 min. The individual fatty acid content was expressed a peak area percentage of total identified methyl esters.
Statistical evaluation
In order to get an idea of the average hoof horn quality of the horses investigated in this work, the average of all marks concerning the hoof horn quality was formed. Furthermore, the mean value of the marks within various statistical groups was formed, where a distinction was made between gender, race or age. In a next step, the subjects were divided according to their HQ/HM scores in five (grade 1-5 HQ, group A-E) or three (HM: moist, dry, balanced; group X-Z) groups. In order to test a relation between age and fatty acid pattern subjects were allocated to four categories: I: ≤ 5 years; II: 6-10 years; III: 11-15 years; IV: ≥ 16 years.
The results obtained from the gas chromatography were given as a percentage of each fatty acid from the total fatty acid content of the Stratum corneum. The respective averages, standard errors and significance levels were formed, the latter being carried out over a two-sided t-test, because the record could be considered to be normally distributed. Because the groups contained of differently sized sample sizes with homogeneous variance the t-test type 2 has been chosen. The statistical analysis was performed through the Student's t-test by the program R i386. In order to minimize the risk of a wrong decision provoked by the t-test, a p-value ≤ 0.01 has been chosen. Due to the high individual variability of fatty acid pattern in Stratum corneum we chose 0.01 ≤ p<0.05 just as trend to significance. The t-test was each performed for two statistical groups in comparison (e.g., pX/Y, pX/Z, pY/Z).
Results
In this study, 30.43% of the subjects had an average hoof horn (mark 3). The marks 1 (excellent), 2 (good), 4 (poor) and 5 (insufficient) are equally distributed, each with 17.39% (not included). Neither in regard of gender nor of age has significant difference in the hoof horn quality detected (Figure 1). Due to the small sample size within the individual breeds, no statement about a relationship between hoof horn quality and breed was examined.
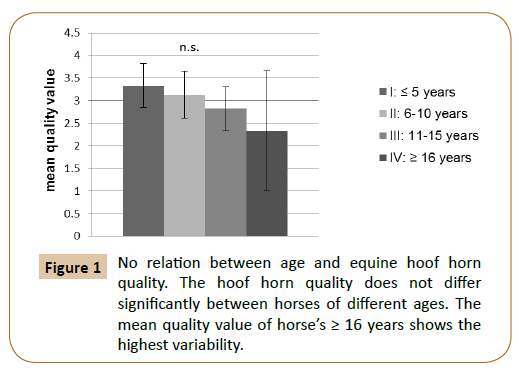
Figure 1: No relation between age and equine hoof horn quality. The hoof horn quality does not differ significantly between horses of different ages. The mean quality value of horse’s ≥ 16 years shows the highest variability.
High variability of fatty acid pattern in the stratum corneum of equine hoof horn
Using gas chromatography, a total of 26 fatty acids was identified in the hoof horn of each tested horse (Table 3). Stearic acid (C18:0) with a percentage of 23.72 ± 1.36% of total fatty acid amount is the predominant fatty acid in the equine hoof horn. This is followed by palmitic acid (C16:0) with 15.05 ± 0.53%, oleic acid (C18:1) with 15.85 ± 1.16% and linoleic acid (C18:2) with 8.86 ± 0.77%. Caproic acid (C6:0), caprylic acid (C8:0), capric acid (C10:0), undecylic acid (C11:0), lauric acid (C12:0), tridecanoic acid (C13:0), cis-10- pentadecylic acid (C15:1), arachidonic acid (C20:0) and heneicosanoic acid (C21:0) were found only in extremely small amounts of less than one percent. All other fatty acids were with 1.0-5.0% in the medium range. Moreover, an individually associated high variability of fatty acids was found in terms of their percentage by mass of the hoof horn. Accordingly, myristic acid with 186.85% showed the largest variability (not shown) in the hoof horn. This is followed by caproic acid (C6:0) with a variance of 93.92%, pelargonic acid (C9:0) with 71.67%, linolenic acid (C18:3) with 86.82% and heneicosanoic acid with 72.61%. All other fatty acids showed mass content variances of 17-50% variance and thus falling below the average variance of 54%. The lowest variability had the palmitic acid (C16:0) with 17.01%, of which percentage lied the total mass of between 10% and 18%.
| Fatty acid |
Mean in % |
SEM |
| C6:0 |
0.156637 |
0.030675 |
| C8:0 |
0.227877 |
0.01992 |
| C9:0 |
2.316159 |
0.346129 |
| C10:0 |
0.160117 |
0.014915 |
| C11:0 |
0.086953 |
0.008231 |
| C12:0 |
0.383811 |
0.033262 |
| C13:0 |
0.223911 |
0.019628 |
| C14:0 |
2.042178 |
0.154544 |
| C14:1 |
1.052565 |
0.410095 |
| C15:0 |
1.560967 |
0.108766 |
| C15:1 |
0.682296 |
0.060205 |
| C16:0 |
15.05264 |
0.533799 |
| C16:1 |
2.075218 |
0.15717 |
| C17:0 |
3.028199 |
0.222741 |
| C17:1 |
2.003745 |
0.208739 |
| C18:0 |
23.7191 |
1.357079 |
| C18:1 |
15.84742 |
1.157097 |
| C18:1t |
2.851073 |
0.132723 |
| C18:2 |
8.855684 |
0.768453 |
| C18:3 |
1.303666 |
0.236005 |
| C20:0 |
1.806615 |
0.100492 |
| C20:1 |
0.69844 |
0.076624 |
| C21:0 |
0.258587 |
0.039151 |
| C22:0 |
3.1983 |
0.275406 |
| C22:1 |
1.518106 |
0.141632 |
| C24:0 |
3.995068 |
0.280913 |
Table 3: Fatty acid pattern in Stratum corneum of equine hoof horn.
Age specificity of fatty acid pattern in the stratum corneum
This study shows an age specific mass fraction of margaric acid (C17:0) in Stratum corneum. 16 to 10 year old animals showed with 3.8 ± 0.55% a significantly higher mass fraction of these fatty acid than younger animals with 2.08 ± 0.16% (p=0.005*) and 11 to 15 year old with 2, 9 ± 0.26% (p =0.009*). The hoof of horses that are 16 years and older, had 3.15 ± 0.63% mass fraction of margaric acid (Figure 2).
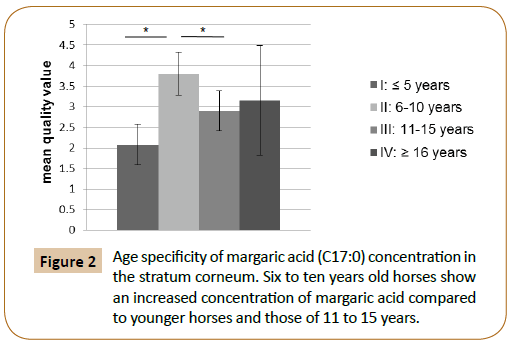
Figure 2: Age specificity of margaric acid (C17:0) concentration in the stratum corneum. Six to ten years old horses show an increased concentration of margaric acid compared to younger horses and those of 11 to 15 years.
Relation between hoof horn quality and fatty acid composition in the Stratum corneum
It has been examined to what extent a relation between the fatty acid content of individual fatty acids and the quality of the hoof capsule existed. The gas chromatographic fatty acid analysis of the hoof horn showed that the hoof horn quality of the horse is reflected in different mass fractions of the fatty acids myristic acid (C14:0), palmitoleic acid (C16:1) and margaric acid (C17:0) (Figures 3 and 4; Table 4A). Thus, a high quality hoof horn (grade 1) was characterised by an increased content of margaric acid compared with poor quality hoof horn (p=0.0099*). Hoof of insufficient quality (grade 5), however, showed in comparison to a good quality hoof (grade 2) a reduction in palmitoleic acid (p=0.004*) and myristic acid (p=0.003*) (Table 4B).
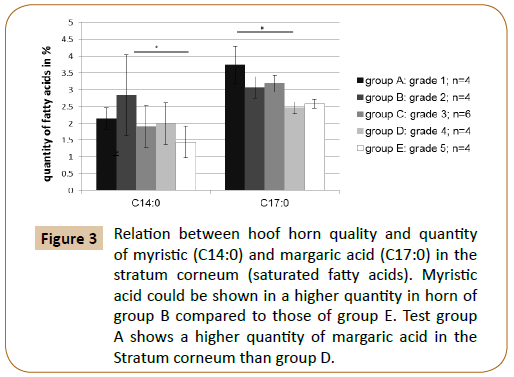
Figure 3: Relation between hoof horn quality and quantity of myristic (C14:0) and margaric acid (C17:0) in the stratum corneum (saturated fatty acids). Myristic acid could be shown in a higher quantity in horn of group B compared to those of group E. Test group A shows a higher quantity of margaric acid in the Stratum corneum than group D.
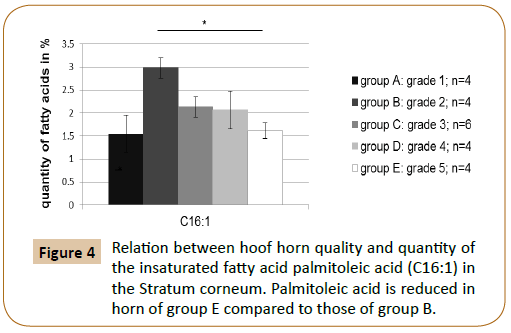
Figure 4: Relation between hoof horn quality and quantity of the insaturated fatty acid palmitoleic acid (C16:1) in the Stratum corneum. Palmitoleic acid is reduced in horn of group E compared to those of group B.
| |
|
|
SEM |
|
|
|
| |
group A; n=4 |
group B; n=4 |
group C; n=6 |
group D; n=4 |
group E; n=4 |
|
| |
mark 1 |
mark 2 |
mark 3 |
mark 4 |
mark 5 |
|
| C6:0 |
0.294458 |
0.221148 |
0.121103 |
0.065347 |
0.10778 |
|
| C8:0 |
0.262154 |
0.250577 |
0.24338 |
0.174872 |
0.196775 |
|
| C9:0 |
1.028255 |
3.355642 |
2.591359 |
3.294446 |
1.104691 |
|
| C10:0 |
0.14054 |
0.183139 |
0.191971 |
0.143379 |
0.117664 |
|
| C11:0 |
0.117683 |
0.080667 |
0.08552 |
0.066307 |
0.085666 |
|
| C12:0 |
0.287438 |
0.487737 |
0.366813 |
0.386939 |
0.402876 |
|
| C13:0 |
0.238039 |
0.2962 |
0.197479 |
0.22127 |
0.186393 |
|
| C14:0 |
2.137522 |
2.839615 |
1.905994 |
1.985166 |
1.444733 |
p*(B/E)=0.003 |
| C15:0 |
1.576953 |
2.055931 |
1.343004 |
1.510507 |
1.481915 |
|
| C16:0 |
16.09371 |
16.01796 |
14.85907 |
13.98693 |
14.4507 |
|
| C17:0 |
3.740871 |
3.057964 |
3.185034 |
2.459301 |
2.5802 |
p*(A/D)=0.0099 |
| C18:0 |
24.42874 |
17.87292 |
24.50522 |
23.46806 |
27.73099 |
|
| C20:0 |
2.072596 |
1.379111 |
1.859949 |
1.610684 |
2.070732 |
|
| C21:0 |
0.272631 |
0.227152 |
0.240701 |
0.146365 |
0.419501 |
|
| C22:0 |
4.486843 |
3.27654 |
2.880612 |
2.345819 |
3.23995 |
|
| C24:0 |
4.401499 |
3.126434 |
4.477926 |
2.913639 |
4.693697 |
|
| |
|
|
SEM |
|
|
|
| |
group A; n=4 |
group B; n=4 |
group C; n=6 |
group D; n=4 |
group E; n=4 |
|
| |
mark 1 |
mark 2 |
mark 3 |
mark 4 |
mark 5 |
|
| C6:0 |
0.103608 |
0.083404 |
0.044942 |
0.031372 |
0.053967 |
|
| C8:0 |
0.059121 |
0.04587 |
0.044436 |
0.020558 |
0.053134 |
|
| C9:0 |
0.324702 |
1.196993 |
0.613966 |
0.626667 |
0.473821 |
|
| C10:0 |
0.043545 |
0.029324 |
0.034468 |
0.005671 |
0.037906 |
|
| C11:0 |
0.016389 |
0.01705 |
0.015539 |
0.017122 |
0.028426 |
|
| C12:0 |
0.116034 |
0.038183 |
0.050255 |
0.035311 |
0.12616 |
|
| C13:0 |
0.053507 |
0.077217 |
0.030335 |
0.031775 |
0.031003 |
|
| C14:0 |
0.566493 |
0.32739 |
0.247508 |
0.179806 |
0.130453 |
|
| C15:0 |
0.238741 |
0.189606 |
0.293715 |
0.074973 |
0.170499 |
|
| C16:0 |
0.610888 |
0.514901 |
1.278459 |
1.605074 |
1.644382 |
|
| C17:0 |
0.088194 |
0.319021 |
0.413196 |
0.333823 |
1.000062 |
|
| C18:0 |
1.840899 |
1.496348 |
2.300318 |
6.097716 |
1.290439 |
|
| C20:0 |
0.343677 |
0.17131 |
0.190945 |
0.212968 |
0.03763 |
|
| C21:0 |
0.170903 |
0.011422 |
0.048174 |
0.03922 |
0.115691 |
|
| C22:0 |
1.046979 |
0.44472 |
0.433863 |
0.578117 |
0.384986 |
|
| C24:0 |
0.903327 |
0.550023 |
0.550152 |
0.387983 |
0.394101 |
|
Table 4a: Relation between quality of the hoof horn and content of saturated fatty acids.
| |
|
|
Mean in % |
|
|
|
| |
group A; n=4 |
group B; n=4 |
group C; n=6 |
group D; n=4 |
group E; n=4 |
|
| |
mark 1 |
mark 2 |
mark 3 |
mark 4 |
mark 5 |
|
| C14:1 |
0.30824 |
0.449709 |
1.79348 |
0.27824 |
1.877472 |
|
| C15:1 |
0.561379 |
1.031799 |
0.659669 |
0.43324 |
0.742363 |
|
| C16:1 |
1.542212 |
2.97789 |
2.132163 |
2.066075 |
1.615039 |
p*(B/E)=0.004 |
| C17:1 |
2.485607 |
2.919769 |
1.537903 |
1.60125 |
1.823576 |
|
| C18:1 |
14.76956 |
17.14515 |
13.85002 |
21.51714 |
13.45328 |
|
| C18:1t |
2.761924 |
3.062847 |
2.518908 |
3.26905 |
2.89176 |
|
| C18:2 |
7.64642 |
9.267207 |
9.701397 |
8.765456 |
8.263657 |
|
| C18:3 |
0.68714 |
1.005283 |
1.690453 |
1.408106 |
1.43726 |
|
| C20:1 |
0.706032 |
0.49261 |
0.729993 |
0.904067 |
0.635835 |
|
| C22:1 |
1.396944 |
1.747823 |
1.405959 |
1.320578 |
1.803334 |
|
| |
|
|
SEM |
|
|
|
| |
group A; n=4 |
group B; n=4 |
group C; n=6 |
group D; n=4 |
group E; n=4 |
|
| |
mark 1 |
mark 2 |
mark 3 |
mark 4 |
mark 5 |
|
| C14:1 |
0.053341 |
0.04709 |
1.077457 |
0.052299 |
1.59632 |
|
| C15:1 |
0.063204 |
0.179904 |
0.091882 |
0.133113 |
0.075275 |
|
| C16:1 |
0.409711 |
0.297329 |
0.221165 |
0.411667 |
0.174156 |
|
| C17:1 |
0.724852 |
0.624614 |
0.279006 |
0.107632 |
0.357919 |
|
| C18:1 |
0.41951 |
1.04703 |
1.942854 |
4.951309 |
1.855351 |
|
| C18:1t |
0.248578 |
0.209196 |
0.216383 |
0.42535 |
0.418445 |
|
| C18:2 |
1.136053 |
0.916859 |
2.115656 |
1.403525 |
2.508085 |
|
| C18:3 |
0.218673 |
0.180165 |
0.605994 |
0.433316 |
0.851855 |
|
| C20:1 |
0.129914 |
0.193796 |
0.218147 |
0.110917 |
0.089131 |
|
| C22:1 |
0.168778 |
0.325909 |
0.299643 |
0.2203 |
0.568599 |
|
Table 4b: Relation between quality of the hoof horn and content of saturated fatty acids.
Relation between moisture of the hoof horn and fatty acid composition in the stratum corneum
Finally the relation between hoof horn moisture and the fatty acid composition in the stratum corneum has been investigated. The results are shown in Table 5 and Figure 5. It is obvious that very moist hoof horn had a significantly higher content of caprylic acid (p=0.0066*) and heneicosanoic acid (p=0.0003***) than very dry horn. Dry hoof horn also showed a significantly lower undecylic acidity than horn with balanced moisture (p=0.006*).
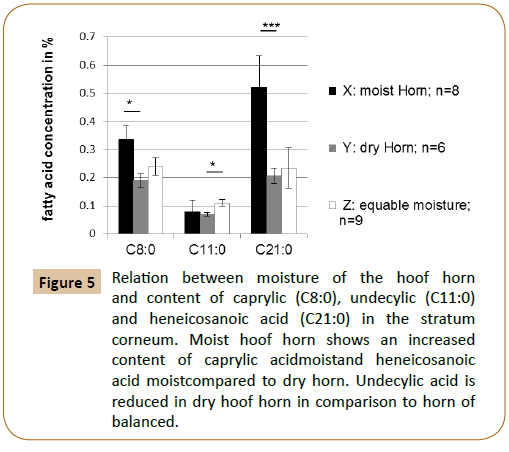
Figure 5: Relation between moisture of the hoof horn and content of caprylic (C8:0), undecylic (C11:0) and heneicosanoic acid (C21:0) in the stratum corneum. Moist hoof horn shows an increased content of caprylic acidmoistand heneicosanoic acid moistcompared to dry horn. Undecylic acid is reduced in dry hoof horn in comparison to horn of balanced.
Discussion
The results of this study confirm that the subjectively rated hoof horn quality of the domestic horse is in no way influenced by the age and sex of the animal. This confirms previous studies [16-18].
Earlier studies showed a breed specific horn quality [7,9,19]. This could not be proven in the present work, because just one breed was represented by more than one individual.
The results of this work show that the overall fatty acid composition in the Stratum corneum of the equine hoof horn is analogous to the one in human skin, where stearic, palmitic, oleic and linoleic acids are the predominant fatty acids [12,20]. High inter individual variances of the fatty acid compositions have already been shown in previous studies and were described as multifactorial [21].
Margaric acid from hoof fat can be soaken up by hoof horn
The analysis of fatty acid composition in the Stratum corneum does not refer to any gender specificity, but to age specificity for margaric acid. Margaric acid could yet be detected in nature only in small quantities and is mainly found in butter [22], which in turn is often used in the manufacture of soaps and waxes. The hooves of the subjects were maintained on a regular basis after training with hoof wax and fat. Ingredients of hoof waxes are not well described. Nevertheless, most wax products (eg. human cosmetics) contain stearic acid, oleic acid and palmitoleic acid [23,24]. Cosmetic fats are made of butterfat including for the most part margaric acid [25]. It is not unlikely that the lipidcontaining intercellular kit of Stratum corneum [11] has soaken up that fatty acid. The age-specific difference of margaric acid quantity can be explained as follows: Sport horses achieve their physical maximum of power at the age of six to ten years. These animals are trained more regularly than younger or older horses and their hooves therefore are often greased after training, so that in the end, more margaric acid can be absorbed by the hoof. This shows the high absorption capacity of the hoof horn against fatty acids from the environment. The owners/trainers of subjected horses favoured the hoof fat, not the wax. Fat contains less or no stearic acid, oleic acid and palmitoleic acid. This fact explains the age specific difference in margaric acid quantity, but not in stearic acid, oleic acid and palmitoleic acid.
The equine hoof horn quality depends on the fatty acid composition in the Stratum corneum
In order to check whether a relation between the fatty acid composition in the Stratum corneum and the hoof horn exists, the subjects were divided into five groups according to their hoof horn quality and their fatty acid profile compared to one another. The results show that a relationship exists between the hoof horn quality and the content of myristic acid, margaric acid and palmitoleic acid.
Hoof horn of insufficient quality shows a reduction of palmitoleic acid and myristic acid. Palmitoleic acid and myristic acid could be identified as an important component of the Stratum corneum in previous studies [12]. Previous studies have already shown that the claw horn of cows with laminitis contain significantly lower amounts of palmitoleic acid than the claw horn from healthy animals [26]. This suggests that palmitoleic acid as the main constituent of the Stratum corneum has a significant effect on the quality of the hoof- and claw horn, which in turn protects the distal limb from diseases caused by external influences [27]. The fact that hoof horn of excellent quality has a reduced content of this fatty acid indicates that besides the palmitoleic acid concentration, other factors play a major role [28]. Myristic acid plays a major role in the structure of the Stratum corneum [12]. As a highly lipophilic fatty acid it can be assumed, that myristic acid plays an important role in maintaining the hoof horn impermeable for water and bacteria. Previous studies showed that the horn of poor quality showed a weaker permeability barrier than the horn of good quality [3]. In addition, it could be demonstrated that myristic acid generally plays a decisive role in the post-translational acylation of proteins [29]. Since these proteins were detected mainly in the cytosol, it is believed that a myristic acylation may be of importance with respect to certain protein-protein or protein-substrate interactions [29]. Similar to the palmitoylation [30], the myristicylation could thus bring about a stabilization of proteins and promote the interactions between lipids and proteins in cell membranes. The way of protein interactions, which are crucial in terms of hoof horn quality, can be diverse. For example, the activity of hydrolases could be regulated, which contribute to the lysosomal function of cementing substance in the hoof [31] and protect the hoof against microbial influences. Myristic acid would then contribute to hoof horn quality indirectly. On the other hand, excellent hoof horn has a higher proportion of margaric acid than such of poor quality. As already mentioned, margaric acid often occurs in fats and waxes [23-25]. So it can also get in the hoof horn with the horn care. If margaric acid actually improve the horn quality, a targeted Horn care using certain waxes or creams could be beneficial. Horses from conventional livestock housing can’t use the water regulation capacity, as is the case with wild living equides sufficiently [32]. Here the watering of the hooves and the subsequent sealing with a suitable fat/wax can reduce the risk of dehydration.
The equine hoof moisture is directly related to the fatty acid composition in the Stratum corneum
Both moisture and drying out can change the mechanical properties of the hoof horn [33-37]. Here, a permanently moist environment softens the hoof capsule and makes them susceptible to erosion, whereas an increased dehydration of the horn leads to brittleness [10]. It was shown in this context that healthy hoof will not be affected by persistent drought, mud or poor litter, whereas horn of poor quality is much more exposed to these environmental influences [38,39]. It can be assumed that the subjectively rated moisture of the hoof horn has also multifactorial causes, but is significantly influenced by the lipidrich water repellent intercellular kit in the Stratum corneum. Analogous to the hoof horn quality it has been examined in this context, to which extent a link between moisture of the hoof horn and the fatty acid composition of the Stratum corneum could be.According to this, a new grouping of the examined subjects was made in accordance to the subjectively assessed moisture of their hoof horn.
The analysis showed that the content of caprylic acid and heneicosanoic acid is significantly higher in very moist hoof horn than in dry. Dry horn also shows a significantly lower content of undecylic acid as a hoof with balanced moisture. As a naturally occurring fatty acid in human sweat, it has the highest fungitoxicity of all other fatty acids of the C7 to C18 series. In conjunction with the results of the present work it can be concluded that undecylic acid contributes to good hoof horn quality through its protection against microbial influences. Compared to the established theory that undecylic acid has a crucial contribution towards the microbial protection of the hoof horn [33-36] it can be assumed that dry hoof is predisposed for infections: With a balanced water content, natural cracks and crevices are in the intercellular space parallel to the ground border and thus protect the living tissue. This kind of protective mechanism fails in an excessively high or low water content of the hoof horn, so, that, under the same load, cracks are parallel to the longitudinal axis of tube longitudinal axis, so vertically towards the ground border. These cracks can be colonized by microorganisms [40]. Also if there is a reduced microbial protection of the hoof capsule because of the reduced undecylic acid, pathogens can pass through the weakened acid barrier and cause inflammation inside the hoof. The content of undecylic acid therefore seems to be an important factor in maintaining the health of the hoof. A targeted local treatment with this fatty acid could affect the mechanical properties of the hoof capsule considerably for the better.
Previous studies showed breed specificity with respect to the horn quality [36,41]. However, in this study a breed dependent specificity of HQ and HM could not be shown, due to the fact that every breed was represented by only one individual. Furthermore, in this study several fatty acids could not be identified by gas chromatography, but show a significant HQ- and HM- dependent concentration in Stratum corneum. Further studies should determine that lacking knowledge.
Conclusion
This study should be an introduction to a research that studies the effects of endogenous multifactorial hoof horn quality (HQ) and hoof horn moisture (HM) to create a hoof capsule, which is optimally adapted to the strains in the equestrian sport. In the course of this work it was shown that the content of certain fatty acids in the Stratum corneum is related to the subjectively assessed hoof horn quality and hoof horn moisture. A key factor appears to be the myristic acid content. As an important component of the intercellulat kit in Stratum corneum, in which it could be an important component protecting against excessive water inlet/water outlet and antimicrobial activity, it is reduced in hoof horn of insufficient quality [12]. The content of palmitoleic acid and margaric acid is also related to the quality of the hoof horn. Furthermore, the hoof horn moisture is connected to the content of caprylic acid, undecylic acid und heneicosanoic acid. The results of our study showed that fatty acids may have an influence on hoof horn quality; this can be used to select a horse for sport activities or improve the hoof horn quality by supplying fatty acids via diet or hoof fats. Further studies should be performed to investigate the influence of different diets or hoof fats on the fatty acids composition and on this way on the hoof horn quality.
References
- Mulling C, Budras KD (1998) The Intercellular Liquid Membrane Coating Material MCM in the epidermis of the cattle cattle. Wien Tierärtzl Monatsschr 85: 216-223.
- Mülling C (1993) Structure, keratin and horn quality in bales, sole and white line of cattle claw and their importance for claw disease. Free University of Berlin, Berlin, Germany.
- Kempson S, Campbell EH (1998) A permeability barrier in the dorsal wall of the equine hoof capsule. Equine Vet J 26: 15-21.
- Albarano T, Warzecha C (1994) The influence of certain environmental factors on hoof horn strength in cattle and pigs. Anat Histol Embryol 23: 41.
- Frohnes AK, Budras KD (2001) Endogenous influence factors on the horn quality in the soles and bales of the horse's hoof. Horsemanship 17: 437-443.
- Anthauer K (1996) The segment-specific structure of the intercellular carcass in the hoof epidermis of the horse. Free University Berlin, Berlin, Germany.
- Josseck H (1995) Hoof horn abnormalities in Lipizzaner horses and the effect of dietary biotin on macroscopic aspects of hoof horn quality. Equine Vet Journal 27: 175-182.
- Ley WB, Pleasant RS, Dunnington EA (1998) Effects of season and diet on tensile strength and mineral content of the equine hoof wall. Equine Vet J 26: 46-50.
- Tscherne L (1910) On the relation of the quality of the wall horn of the horse's hooves to the histological device. Free University of Berlin, Berlin, Germany.
- Baggott DG (1982) Hoof lameness in dairy cattle. In Practice 4: 133-141.
- Scaife J, Meyer K, Grant E (2000) Comparison of the lipids of the bovine and equine hoof horn. Proc III Conf on Bovine Lamenesses.
- Schneider IM, Wohlrab W, Neubert R (1997) Fettsäuren und Epidermis. Der Hautarzt 48: 303-304.
- Pütz AC (2007) Monitoring of seasonal, behavioral and domestication-induced influences on the horn quality of the horse's hoof. Free University of Berlin, Berlin, Germany.
- Hayward AF (1979) Membrane coating granules. Int Review Cytol 59: 97-106.
- Landmann L (1988) The epidermal permeability barrier. Anat Embryol 178: 1-3.
- Reily JD, Collins SN, Cope BC, Hopegood L, Latham RJ (1998) Tubule density in the stratum medium of horse hoof. Equine Vet J 26: 4-9.
- König B (2001) Structure, function and quality of the Kronhorn in the horse's hoof. Free University of Berlin, Berlin, Germany.
- Patan B (2001) Seasonal influence on the horn formation rate, horn abrasion and horn quality in the hoof wall of Przewalskipferden (Equus ferus przewalskii). Free University of Berlin, Berlin, Germany.
- Zenker W (1991) Hoofhorn changes in Lipizzan horse’s u a treatment experiment with biotin. Free University of Berlin, Berlin, Germany.
- Lampe M, Burlingame A, Whitnes AL, Williams J, Brown ML, et al. (1983) Human stratum corneum lipids: characterization and regional variations. J Lipid Res 24: 120-130.
- Pütz AC (2007) Monitoring of seasonal. Attitude and domestication-induced influences on the horn quality of the horse shoe. Free University of Berlin, Berlin, Germany.
- Hansen RPF, Shorland B, June Cooke N (1957) Occurrence in Butterfat of n-Heptadecanoic Acid (Margaric Acid). Nature 179: 98.
- Daniel C (2004) Patent: Acetylated wax compositions and articles containing them. US 20060075679 A1.
- John E (2013) Patent: Wax Compositions including a slip agent. WO 2013155314.
- Hansen RP, Shorland FB, Cooke NJ (1957) Occurrence in Butterfat of n-Heptadecanoic Acid (Margaric Acid). Nature 179: 98.
- Offer JE, Logue DN (1998) The effect of lameness in the dairy cow on the fatty acid profile of claw horn lipids. Proceedings: 10th International Symposium on Lameness in Ruminants, Casino Lucerne, Switzerland.
- Zietzschmann O (1913) The structure and nomenclature of the hoof-skin parts. Berl Tierärztl Wschr 35: 626-628.
- Reilly JD (1995) No Hoof no horse?. Equine Vet J 27: 166-168.
- Magee AI, Courtneidge SA (1985) Two classes of fatty acid acylated proteins exist in eukaryotic cells. The EMBO J 4: 1137-1144.
- Linder ME, Deschenes RJ (2007) Palmitoylation: policing protein stability and traffic. Nature Rev Mol Cell Biol
- 8: 74-84.
- Budras KD, Bragulla H (1991) Special features of the membrane coating material (MCM) between the hard horns of the horse's hoof. Anat Anz (Suppl) 170: 435-446.
- Budras KD, Patan B, Mülling CH (2002) Scanning and transmission electron microscopy and physical measurements of the water binding capacity at the saumhorn of the horse's hoof. Wien Tierärtzl Mschr 89: 180-187.
- Dolezalova M, Janis R, Svobodova H, Kasparkova V, Humpolicek P, et al (2010) Antimicrobial properties of 1-monoacylglycerols prepared from undecanoic (C11:0) and undecenoic (C11:1) acid. Europ J Lipid Sc Techn 10: 1106-1114.
- Garg AP, Müller J (1993) Fungitoxicity of fatty acids against dermatophytes. Mycoses 36: 51-63.
- Zeng XN, Leyden JJ, Spielmann AI, Petri G (1995) Analysis of characteristic human female axillary odors: Qualitative comparison to males. J Chem 22: 237-247.
- Ammendola S, Lembo A, Battistoni A, Tagliatesta P, Ghisalberti C, et al. (2009) 10-Undecanhydroxamic acid, a hydroxamate derivative of the undecanoic acid, has strong antimicrobial activity through a meachnism that limits iron availability. FEMS Microbiol Letters 294: 61-67.
- Tscherne L (1919) On the relationship of the quality of the horseshoe of the horse's hooves to the histological device. Free University of Berlin, Berlin, Germany.
- Eustance RA (1994) Factors affecting equine hoof horn growth rate and quality. In Practice 16: 129-140.
- Kempson SA (1990) Ultrastructural observations on the response of equine hoof defects to dietary supplementation with Farriere’s Formula. Vet Rec 127: 494-498.
- Bertram JEA, Goslone JM (1987) Functional design oh horse hoof keratin: The modulation of mechanical properties through hydration effects. J Exp Biol 130: 121-126.
- Josseck H (1995) Hoof horn changes in Lipizzan horses and a treatment experiment with biotin. Investigation of macroscopic hoof status and horn growth on the course of plasma biotin levels on the genetic basis of hoof horn damage. Equine Vet J 27: 175-182.
- Zenker W (1991) Hoof horn changes in Lipizzan horses and a treatment experiment with biotin. Equine Vet J 27: 175-182.