Nazik Z Eisa1, Salih A Babiker2 and Hamid S Abdalla3
1Department of Animal Production, Faculty of Agriculture, University of Gezira, Wad Medani, Sudan
2Department of Meat Production, Faculty of Animal Production, University of Khartoum, Khartoum, Sudan
3Department of Parasitology, Faculty of Veterinary Sciences, University of Khartoum, Khartoum, Sudan
Corresponding Author:
Nazik Z Eisa
Department of Animal Production
Faculty of Agriculture, University of Gezira, Wad Medani, Sudan
Tel: 2348135655346
E-mail: eisanazik@uofg.edu.sd
Received Date: March 28, 2017; Accepted Date: April 06, 2017; Published Date: April 13, 2017
Citation: Eisa NZ, Babiker SA, Abdalla HS. Effect of Natural Gastrointestinal Parasitic Infection on Fattening Performance of Sudan Desert Sheep. J Anim Sci Livest Prod. 2017, 1:1.
Copyright: © 2017 Eisa NZ, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Sudan desert sheep; Natural infection; Gastrointestinal parasites (GP); Feedlot performance
Introduction
The livestock population of Sudan, amount to 114 million head of which the sheep comprise about 40 million head (According to the Ministry of Livestock, Fisheries and Range lands of the Sudan 2016). Desert sheep comprise about 80% of the slaughtered sheep in Sudan [1] which greatly contributes in Sudan economy. Worldwide, gastrointestinal nematodes represent a major production problem for ruminant [2]. Energy level of the diet was suggest by experts as one of the factors that affect infestation by internal parasites [3]. Jason, et al. [4] stated that sheep affected by worms show reduced appetite for pasture and reduction in feed intake. Inadequate and poor quality feeds are often a major constraint to small ruminant production in smallholder systems [5]. An improved nutritional status may reduce, under some conditions, the production losses and mortality rates associated with gastrointestinal nematode infections [6,7]. Poorly nourished animals are more susceptible to infection with parasites. This could be due to a lack of overall protein and energy or a poorly balanced diet, deficient in minerals [4]. Supplementation is a common practice in several ruminant production systems [8] can cause a reduction in voluntary feed intake in housed ruminants [9]. Cardia et al. [10] stated that sheep gastrointestinal infection causes severe reduction in daily weight gain and demonstrates awful food conversion.
Materials and Methods
Animals
Forty-eight naturally infected Sudan Desert sheep (Hamary type) with an average initial live weight of 24.13 kg ± 1.22 kg were used for this study. The study was conducted at the Department of Animal Nutrition, Faculty of Animal Production, University of Khartoum, Khartoum North, Sudan. After screened for internal and external parasites, all animals were treated with cypermethrin (ectothrin) against external parasites while half of them were treated for internal parasites as the control and the rest were left as naturally infected. Antibiotic injections (Oxytosin-10%) were given for protection against respiratory diseases and coccidiobans against coccidiosis. Half of the control group (12 out of 24) were less than one year old (milk teeth) and the other half were two years old. Half of the group were supplied with high energy diet while the rest were given low energy diet. Same applied for the naturally infected group ending up with 8 groups (6 animals each) with equal average weight. Each two animals were kept in a pen provided with water, feed and salt lick cubes.
Experimental design
Health, age and dietary energy level are the three parameters on which the design depends. At the end of the adaptation period individual initial weight was taken and animals were randomly divided into two groups of twenty-four animals each. One group was treated for internal parasites while the other group was left naturally infected. Each group was further subdivided into four subgroups of six animals each. Further subgrouping was done through keeping each two animal in a pen provided with water and feed facilities. Each group was divided in to two sub-groups according to age, old (2 years) and young (one year), dietary energy (high and low), and health (treated and naturally infected). The design ended up with 8 groups which were, old treated high energy (OTHE), old infected high energy (OIHE), old treated low energy (OTLE), old infected low energy (OILE), young treated high energy (YTHE), young infected high energy (YIHE), young treated low energy (YTLE) and young infected low energy (YILE).
Determination of parasites and anthelmintic application
Upon arrival animals were screened for parasites and this was done weekly throughout the adaptation and experimental periods. At the start of the experiment all experimental animals were treated for external parasites by using Ectothrin dipping solution. Internal parasites Anthelmintics were applied in the very beginning to treat the group of animals need to be treated, then for the rest of the experiment for the treated group as prevention. Coccidiobans were given for all experimental animals for treatment and control of coccidian infection throughout the experimental time. Oxytetracyclin and Gentamycine were used against respiratory tract infections.
Experimental rations
Two iso-nitrogenous diets (CP: 16.11%) of high and low energy were formulated. The ingredients of the experimental diets are given in Table 1.
| Ingredients |
High energy diet (%) |
Low energy diet (%) |
| Sorghum grains |
40 |
4 |
| Wheat bran |
15 |
5 |
| Groundnut cake |
11 |
6 |
| Molasses |
14 |
30 |
| Groundnut hulls |
17.8 |
51.4 |
| Urea |
0.2 |
2.4 |
| Limestone |
1 |
1 |
| Common salt |
1 |
1 |
| *Calculated ME (MJ/Kg) |
12.24 |
10.35 |
| Calculated CP (%) |
16.11 |
16.11 |
| *Calculated according to MAFF [11] |
Table 1: Ingredient proportions of the experimental diets.
Chemical composition of the experimental diets
Table 2 shows the chemical composition of low and high energy diets. Samples of high and low energy diets were analyzed for proximate composition of dry matter (DM), crude protein (CP) using Kieldahl method of analysis, ether extract (EE) using soxhlet apparatus, crude fiber (CF) and Ash according to AOAC [12]. All samples were analyzed in replicates and the means were then taken. The protein percentage in both high and low energy diet was 16.11%.
| Parameter (%) |
High energy Diet |
Low Energy Diet |
| Dry Matter |
91.16 |
89.74 |
| Crude Protein |
16.11 |
16.11 |
| Crude Fiber |
15.31 |
25.17 |
| Ether Extract |
2.04 |
1.36 |
| Nitrogen Free Extract |
50.04 |
33.55 |
| Ash |
2.28 |
8.17 |
Table 2: Chemical composition of experimental diets.
Feeding program
Feed was offered ad lib in the early morning (8.00 am). Feed refusals were collected and weighed every day in the morning before feeding throughout the data collection period. Samples of feed offered and refused were taken weekly for analyzing DM, then pooled to monthly samples for further analysis.
Feed intake
Feed intake of each group was recorded daily and calculated as the difference between the weight of the quantity offered and refusal on the next morning. The average dry matter of the experimental diet and the refusal were obtained to calculate dry matter intake.
Live body weight gain
Initial live body weight was recorded for each animal on the first day of the experiment. Through the experimental period animals were weighed weekly in the morning after an overnight fast except for water using Weigh Bridge balance of 150 kg maximum capacity load with 0.1 kg division.
Statistical analysis
Experimental data were analyzed using the randomized block design of (3 × 3) factorial arrangement. Data were statistically analyzed using analysis of variance pertaining to factorial arrangement by Statistical Package for Social Sciences (SPSS) (Version.15, 2007). Data was analyzed to address the objectives of the study. This comprised descriptive analysis and significant treatment means were compared by Duncan´s multiple range test.
Results
Prevalence of parasites in experimental lambs
Table 3 shows the prevalence of both internal and external parasites in the experimental lambs. All animals were found to be infected with either internal parasites (IP), external parasites (EP) or both of them. Those infected with internal parasites were (96%) while those infected with external parasites were (52%).
| Trait |
Infected
(GP&EP) |
Infected
(GP) |
Ticks
(EP) |
| No. of lambs(48) |
48 |
46 |
25 |
| No. of inf. lambs |
| %of inf. lambs |
100 |
96 |
52 |
In this table and the following ones:
GP: Gastrointestinal parasites
EP: External parasites |
Table 3: Natural infection of experimental animals.
Table 4 shows the first week screening for internal parasites. All lambs were found to be infected with internal and or external parasites. Ninety six percentages of lambs were found to be infected with internal parasites only (Trichostrongyles, Monieza and coccidia). Of the internal parasites Trichostrongyles was the most dominant parasite (80%), followed by Monieza (40%), Coccidia (26%), mixed infection comprised of Trichostrongyles and Monieza (33%), Trichostrongyles and coccidia (31%), Monieza and coccidia (17%) and Trichostrongyles, Monieza and coccidia is (15%).
| Trait |
No. of IL |
% of IL |
| Number of animals=48 |
| IP: Internal parasites |
46 |
96 |
| Trichostrongylus |
38 |
82.6 |
| Monezia |
19 |
41.3 |
| Coccidia |
21 |
45.65 |
| Trichostrongylus+Monezia |
16 |
34.65 |
| Trichostrongylus+Coccidia |
15 |
32.61 |
| Monezia+Coccidia |
8 |
17.39 |
| Trichostrongylus+Monezia+Coccidia |
7 |
15.22 |
| IL: Infected lambs |
Table 4: Prevalence of fecal ova at first week screening for internal parasites.
Feedlot performance
Data related to feedlot performance of the Sudan desert sheep of the two age groups (young and old), fed different dietary energy levels (low and high) and treated for internal parasites or left naturally infected is presented in Table 5.
| |
Old |
Young |
|
| High energy |
Low energy |
High energy |
Low energy |
| Trait |
Treated |
Infected |
Treated |
Infected |
Treated |
Infected |
Treated |
Infected |
SE |
P-Level |
| Initial Weight (kg) |
25.17 |
23.83 |
27 |
24 |
24.5 |
23.67 |
22.67 |
22.17 |
1.22 |
NS |
| Final Weight (kg) |
39.50a |
33.08abc |
34.40bc |
23.50d |
35.83ab |
32.50abc |
27.83c |
21.92d |
2.37 |
0.001 |
| Total Gain (kg) |
14.33a |
9.25abc |
7.4cd |
-0.5 |
11.33ab |
8.83bc |
5.17cd |
-0.25 |
1.64 |
0.001 |
| Daily Gain (kg) |
0.26a |
0.17abc |
0.13cd |
-0.01 |
0.2ab |
0.16bc |
0.09cd |
-0.01 |
0.03 |
0.001 |
| daily Dry matter intake (kg) |
1.41a |
1.29ab |
0.76cd |
0.75d |
1.27ab |
1.03bc |
0.85cd |
0.68d |
0.99 |
0.001 |
| Feed Conversion ratio (kg/kg L. Wt. G.) |
5.42ac |
7.59ac |
5.85ac |
0.00b |
6.35ac |
6.44ac |
9.49c |
0.00b |
1.71 |
0.01 |
P-Level: Level of significance; SE: Standard Error; Means within the row having different super scripts are significantly different; L. Wt. G.: Live weight gain
Table 5: Effect of age, dietary energy level and internal parasites on performance of desert sheep.
The average final live weight of old lambs treated for internal parasites and given high energy diet was (39.5 kg) which was the highest of all the treatments followed by that of young lambs treated for internal parasites and fed high energy diet too (35.8 kg). All infected lamb groups performed lesser average final live weights than their counterpart treated groups. The average final live weight of the group of infected young lambs given low energy diet was 45% lesser than old treated lambs given high energy diet. Average final live weight of infected old lambs raised on low diet was 23.5 kg which was 32% lesser treated young lambs given low energy diet. Total live weight gain (TLG) of old lambs treated for internal parasites and given high energy diet were 5.08 kg higher than that of infected old lambs. The effect of treatment was clear, since the low energy diet given to old lambs that were treated for internal parasites resulted in significantly (P<0.001) high (TLG) which was 7.9 kg higher than that of infected old lambs, in fact the later had lost 0.5 kg of their initial weight. Young lambs treated for internal parasites and fed high energy diet resulted in 2.5 kg higher (TLG) than that of infected young lambs. Young lambs fed low energy diet and treated for internal parasites had gained only 5.17 kg while infected young and lambs fed low energy diet lost (0.25 kg) and (0.5 kg) respectively.
Average daily live weight gain showed the same pattern as total live weight gain. The effects of internal parasites on live weights of experimental lambs are shown in Figure 1. The initial weights were almost the same. Differences in final weights and weight gain were very clear.
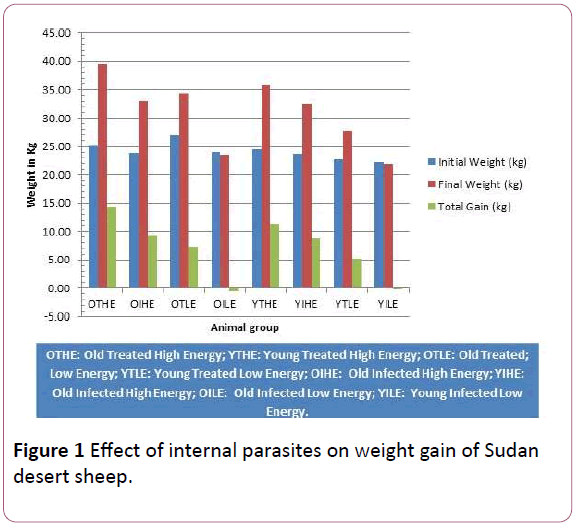
Figure 1: Effect of internal parasites on weight gain of Sudan desert sheep.
Old lambs which were treated for internal parasites and given high energy diet reached the highest final body weight of all experimental groups as well as achieving the highest weight gain, followed by the young lambs which were treated for internal parasites on the same energy level. Infected groups on high energy diet gained more weight than treated groups on low energy diet. On the other hand, infected groups on low energy diet lost weight.
As seen in Table 5 the highest dry matter intake (DMI) was that of old lambs treated for internal parasites and fed high energy diet (1.41 kg) which was 9.3% more than that of the infected old lambs fed on the same energy diet. Young lambs fed low energy diet and treated for internal parasites had a daily (DMI) of 0.85 kg which was higher than that of infected young lambs (0.68 kg). In fact the later performed the least (DMI). In general Figure 2 showed the mean total feed intake of all infected and treated lambs.
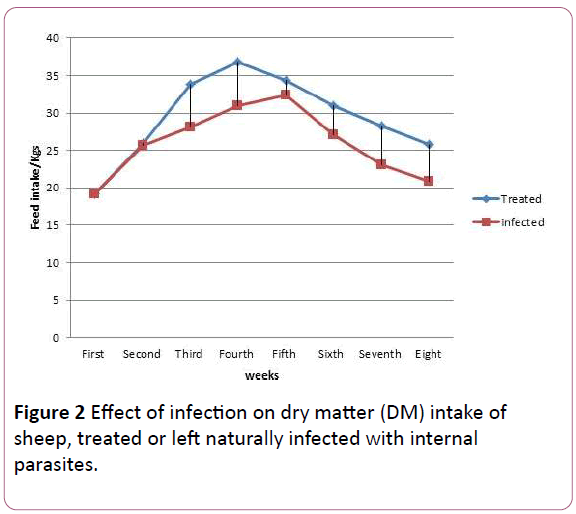
Figure 2: Effect of infection on dry matter (DM) intake of sheep, treated or left naturally infected with internal parasites.
Table 5 revealed that old lambs treated for internal parasites and fed high energy diet had the best feed conversion ratio (FCR)of all groups which was 5.42 kg/kg live weight gain followed by treated old lambs given low energy diet.
Discussion
Growth performance
Changes in body weight are probably the most widely used criterion for judging the effect of gastrointestinal parasitism. In this study, the initial body weight for all lamb groups were not significantly different which allowed fair comparison between groups. The final body weights of old groups were (32.62 kg) which was 10.5% higher than young groups (29.52 kg), irrespective of infection or diet. This result comes along with Zanton et al. [13] who demonstrated that first year infection was negatively related with body weight. Regardless of infection or age, the groups that were fed high energy diets, had higher final body weights than those which were fed low energy diet. The same finding was reported by Kosgey [5] who stated that inadequate and poor quality feeds were a major constraints to small ruminant weight gain. According to Anna [14] this could be due to a lack of overall protein and energy or a poorly balanced diet. Infection with gastro-intestinal parasites had a big impact on final body weight, since the group of lambs which was treated for internal parasites ended up with 34.39 kg final body weight which was higher than those left naturally infected (27.75 kg). This agrees with Dimander et al. [15] and Larsson et al. [16].
It is clearly found in this study, that infestation with gastrointestinal parasites was influenced by age factor when comparing the weight gain of infected old lambs on high energy diets with infected young lambs on the same energy diet (8.83 kg), the older lambs gained more weight (9.25 kg) which could be due to compensatory growth which occurred due to resistance. This view agreed with Richard, et al. [17]. According to this study, young and old lambs on low energy plane of nutrition were more susceptible to the effects of internal parasites than infected lambs on high energy diets. Those on low energy diet suffered severe loss of weight. This might be attributed to the fact that the effect of diet confounded by internal parasites in young animals was more severe than in old ones. In other words, young animals were more susceptible to parasites than older ones which might be fairly resistant. The results obtained by Berry et al. [18] supported this suggestion. This finding came along with the finding of Duval [19] who stated that, age as well as weight of animals determined susceptibility to parasites. Burke and Miller [20] too, stated that young animals and those on a low plane of nutrition are more susceptible to the effects of gastrointestinal parasites.
The effect of energy along with treatment for internal parasites was very clear since the group which was treated and given high energy diet resulted in the highest (39.50 kg) final live weights while that of infected old lambs given the same diet was (33.08 kg). This agreed with Sykes and Coop [6] and Walkden- Brown and Kahn [7] Moreover, young lambs treated for internal parasites and fed high energy diet ended up with (35.83 kg) final live weights while infected young lambs fed the same diet ended up with (32.50 kg). These results agreed with Shaw et al. [21] who proved that grazing lambs artificially and naturally infected with H. contortus and received a supplement consisting of a protein rich feed showed an increase in their resilience and strength but not in their weight gain. Almost an exact result was obtained by Lin et al. [22] who conducted a 63-day experiment studying the production loss due to larval challenge in grazing sheep (given Albendazole) with worm-free sheep. Sheep were either infected with mixed oral infection of albendazolesusceptible (Haemonchus contortus, Trichostrongylus colubriformis and Teladorsagia circumcincta) or remained uninfected. They found that live weight gain was significantly lower in infected un-treated, 27 gm/day and infected treated 55 gm/day than in uninfected untreated, 88 gm/day or uninfected treated 81 gm/day treatments. The effects of infection on production persisted in infected un-treated, but not in infected treated sheep for several weeks after termination of infection. Additionally, Ploeger et al. [23] and Larsson [16] stated that internal parasites infection in sheep during the first grazing season remained up to at least the end of the second grazing season. This suggested that nematode infections occurring in the first 2 years of life negatively influenced meat production by reducing weight gains. It has been shown that nutritional factors, particularly protein, can mitigate against some of the effects of parasitism [24]. Daily dry matter intake of old and young lambs left naturally infected whether fed high or low energy diets was very much less than the intake of lambs treated for internal parasites (Table 3). Holmes [25] reported the same finding that infection of sheep with internal parasites reduced feed intake, accordingly reduced dry matter intake. Holmes [26] earlier found that a depression in voluntary food intake was an important feature of infections with gastrointestinal nematodes. A reduction in intake of up to 77% was recorded by Fox et al. [27] too. Jason [4] also stated that sheep affected by worms showed reduced appetite and feed intake of up to 10% by infections caused by H. contortus and Brown stomach worms. Preston and Willis also indicated that faster gain was associated with high level of feeding. On the other hand, Attaelmanan [28] stated that inadequate and poor quality feeds had great effect on feed intake which coincided with the present finding. This study proved that internal parasites of lambs caused inappetance which varied according to age where younger lamb group lost appetite more than older which coincided with the findings of Forbes et al. [9]. Parasite-induced inappetance has also been suggested for intestinal species of nematode [29]. Kyriazakis et al. [30] proposed many potential functional benefits from parasite-induced anorexia, the most important of which was that, the reduction in intake limited the acquisition of further infections and might also allowed the host to be more selective in its diet. In this study, feed conversion ratio was greatly affected by infestation of lambs with internal parasites, dietary energy level as well as age. Infected lamb groups that were fed high or low energy diets had worse feed conversion ratio than treated lambs whether fed high or low energy diets. This was in line with Cardia et al. [10] who stated that, sheep gastrointestinal tract infection caused severe reduction in daily weight gain and demonstrated awful food conversion. Studies conducted by Soulsby had resulted in the same facts. Depression in feed intake and conversion ratio could be due to disturbances in gastrointestinal motility resulted from gastrointestinal parasitic infection. The same results were obtained by Bueno et al. Feed conversion ratio of old and young lamb groups left naturally infected and fed on low energy diets was very high, since they ate but did not gain any weight. In fact they lost weight. This finding was supported by Wallace et al. and Datta et al. [31].
References
- FAO (2012) Food and Agricultural Organization of the United Nations, Rome, Italy.
- Emmanuel BW (2013) Gastrointestinal parasites in ruminants at selected abattoirs in the Greater Accra region, Ghana.
- Blackburn HD, Paiva SR, Wildeus S, Getz W, Waldron D, et al. (2011) Genetic Structure and Diversity among U. S. Sheep Breeds: Identification of the Major Gene Pools. J AnimSci 89: 2336-2348.
- Jason S, Cameron F, Nicole S (2008) Internal Parasite Control in Sheep. Parasite damage to sheep 11, Australian Sheep Industry CRC.
- Kosgey IS, Van Arendonk JAM, Baker RL (2004) Economic values for traits in breeding objectives for sheep in the tropics. Impact of tangible and intangible benefits. Livest Prod Sci 88: 143-160.
- Sykes AR, Coop RL (2001) Interaction between nutrition and gastrointestinal parasitism in sheep. NZ Vet J 49: 222-226.
- Walkden-Brown SW, and Kahn L (2002) Nutritional modulation of resistance and resilience to gastro-intestinal nematode infection. A review. Asian-Aust J AnimSci 15: 912-924.
- Valderra´bano J, Delfa R, Uriarte J (2002) Effect of feed intake on the development of gastrointestinal parasitism in growing lambs. Vet Parasitol 104: 327-338.
- Forbes AB, Huckle CA, Gibb MJ, Rook AJ,Nuthall R (2000) Evaluation of the effects of nematode parasitism on grazing behaviour, herbage intake and growth in young grazing cattle. Vet Parasitol 90: 111-118.
- Cardia DF, Rocha-Oliveira RA, Tsunemi MH, Amarante AF (2012) Immune response and performance of growing Santa Ines lambs to artificial Trichostrongyluscolubriformis infections. Vet Parasitol 182: 248-258.
- MAFF (Manual of Veterinary Parasitological Laboratory Techniques). London, UK: Her Majesty’s Stationary Office; 1986.
- AOAC (2000) Association of Analytical Chemists, Official methods of analysis (17edn). Association of official analytical chemists. Washington DC.
- Zanton GI, Heinrichs AJ (2005) Meta-analysis to assess effect of prepubertal average daily gain of Holstein heifers on first-lactation production. J Dairy Sci 88: 3860-386
- Anna B (2009) Reducing the risk of internal parasites. Animal welfare Approved Tech No. 4.
- Dimander SO, Hoglund J, Uggla A, Sporndly, E, Waller PJ (2003) Evaluation of gastrointestinal nematode parasite control strategies for first-season grazing cattle in Sweden. Vet Parasitol 111: 193-209.
- Larsson EV (2006) Control of gastrointestinal parasites in first and second-season grazing cattle in Sweden. PhD thesis, University of Agricultural Sciences.
- Richard S, Cabaret J, Cabourg C (1990) Genetic and environmental factors associated with nematode infection in dairy goats in Northwestern France. Vet Parasitol 36: 237-243.
- Berry CL, Dargie JD (1978) The patho-physiology of ovine fascioliasis: the influence of dietary protein and iron on the erythro-kinetics of sheep experimentally infected with Fasciola hepatica. Vet Parasitol 4: 327-339.
- Duval J (1997) The control of internal parasites in cattle and sheep. M. Sc. thesis, McGill University, Canada.
- Miller CM, Waghorn TS, Leathwick DM, Candy PM, Oliver AM, et al. (2012) The production cost of anthelmintic resistance in lambs. Vet Parasitol 186:376-381.
- Shaw KL, Nolan JV, Lynch JJ, Coverdale OR, Gill HS (1995) Effects of weaning, supplementation and gender on acquired immunity to Haemonchuscontortus in lambs. Int J Parasitol 25: 381-387.
- Lin Z, Fernandez-Robledo JA, Cellier MF, Vasta GR (2011) Biochem J 50:6340-6355
- Ploeger HW, Kloosterman A, Rietveld FW, Hilderson H, Berghen P, et al. (1996) Production of dairy replacement stock in relation to level of exposure to gastrointestinal nematode infection in the first grazing season: second-year calves and heifers. Vet Parasitol 65: 99-115.
- Coop RL, Holmes PH (1996) Nutrition and parasite interaction. Int J Parasitol 26: 951-962.
- Holmes PH (1987) Patho-physiology of parasitic infections. J Parasitol 94: 29-51.
- Holmes PH (1985) Pathogenesis of Trichostrongylosis. Vet Parasitol 18: 89-101.
- Fox MT, Gerrelli D, Pitt SR, Jacobs DE, Gill M, et al (1989) Ostertagiaostertagi infection in the calf: effects of a trickle challenge on appetite, digestibility, rate of passage of digesta and live weight gain. Res Vet Sci 47: 294-298.
- Attaelmanan BA (2006) Effect of ammoniation and tree foliage supplementation on bagasse NDF degradability, digestibility and rumen fermentation. Ph.D. Thesis, University of Khartoum, Sudan.
- Symons LEA, Hennessy DL (1981) Cholecystokinin and anorexia in sheep infected by the intestinal nematode Trichostrongyluscolubriformis. Int J Parasitol 11: 55-58.
- Kyriazakis I, Tolkamp BJ, Hutchings MR (1998) Towards a functional explanation of the occurrence of anorexia during parasitic infections. AnimBehaviour 56: 265-274.
- Wallace DS, Bairden K, Duncan JL, Fishwick G, Gill M et al. (1995) Influence of soya bean meal supplementation on resistance to Haemonchosis in Hampshire Down lambs. Res Vet Sci 58: 232-237.