Nwoha RIO1* and Anene BM2
1Department of Veterinary Medicine, Michael Okpara University of Agriculture, Umudike, Nigeria
2Department of Veterinary Medicine, University of Nigeria, Nsukka, Nigeria
*Corresponding Author:
Nwoha RIO
Department of Veterinary Medicine
Michael Okpara University of Agriculture
Umudike, Nigeria
Tel: 08030987115
E-mail: rosemarynwoha@yahoo.com
Received date: April 18, 2017; Accepted date: May 05, 2017; Published date: June 12, 2017
Citation: Nwoha RIO, Anene BM.Assessment of Acetylcholinesterase, Creatine Kinase and Lactate Dehydrogenase as Biomarkers of Trypanosoma brucei Infection in Dogs. J Vet Med Surg. 2017, 1:2. doi: 10.4172/2574-2868.100009
Keywords
Acetylcholineesterase; Creatin kinase; Lactate dehydrogenase; Trypanosoma brucei
Introduction
Trypanosomosis is a wasting disease in which there is a slow progressive loss of condition accompanied by increasing anemia and weakness to the point of extreme emaciation, collapse and death often due to heart failure [1]. In dogs, experimental infection of T. brucei produces an acute disease condition similar to that seen in field infections [2]. The disease produces high parasitaemia, moderately to severe anemia and marked changes in the lymphoid system [2]. Tissue invasion of trypanosomes were associated with marked cellular infiltration comprised of lymphoid cells, plasma cells, macrophages and polymorph nuclear leukocytes. The tissue infiltrates results in severe cellular degeneration and focal necrosis resulting in tissue and organ damages. Up till date chemotherapy and chemoprophylaxis has remained the major means of trypanosomosis control in Nigeria [3]. However the effectiveness of the existing trypanocides as a means of control has been curtailed by wide spread multiple drug resistance, lack of alternative therapy, existence of fake drugs and lack of sufficient veterinary services especially in the rural areas [4,5]. Biomarkers are measureable characteristics that reflect the presence or severity of some disease state [6]. It is an indicator of a particular disease state or some other physiological state of an organism [7]. In medicine, biomarkers can be used as a parameter to measure the progress of disease or the effect of treatment [8]. Several biomarkers have been identified for many disease such as serum LDL for cholesterol, blood pressure p53 and MMPs for cancer cases [9,10]. Due to the present challenge in the treatment and control of trypanosomosis it becomes imperative to explore its biomarkers as a parameter to measure the progress and treatment of the disease condition in dogs.
Materials and Methods
Experimental animals
Twelve young mongrel breed of dogs of both sexes aged between 6 to 8 months and weighing between 6.0 to 8.2 kg were used in the study. The animals were kept in clean and disinfected kennel with a fly proof to prevent bite from wild tse-tse fly. They were screened for both blood and gastrointestinal parasites and positive cases treated accordingly and were fed and watered ad lib.
Ethical observation
The care of the animals was in conformity with the guideline for animals’ experimentation of Council for International Organization of Medical Sciences (CIOMS) for biomedical research involving animals. The dogs were humanely cared for and treated throughout the study. They were comfortably housed in properly ventilated kennels in good hygienic condition and provided good and adequate feeding with clean portable drinking water. All authors hereby declare that all experiments have been examined and approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Trypanosome infection
Trypanosoma brucei used in the study was originally isolated from a naturally infected dog presented at the Veterinary Teaching Hospital, University of Nigeria Nsukka. The parasite was isolated and confirmed at the Department of Parasitology and Entomology, University of Nigeria Nsukka. The parasite was maintained in rat from where parasites were collected and inoculated into the experimental dogs.
Experimental design
The dogs were randomly divided into 2 groups of 6 members each.
• GROUP A- was infected with Trypanosoma brucei parasites,
• GROUP B- was the uninfected control.
GP A dogs were infected with estimated number of 2.5 × 106 trypanosomes per dog. The quantity of parasite was estimated using the rapid matching method as described by Herbert and Lumsden [11]. The infection was done intraperitoneally.
Estimation of parasitaemia
Parasitaemia was determined 2 days post infection until establishment of parasitaemia using both the wet mount and the buffy coat techniques as described by Woo [12]. Post establishment of Trypanosoma brucei infection blood samples for evaluation of biomarkers were collected on days 0, 3, 7, 10, 12, and 16 days post-inoculation (PI), through the cephalic vein into plain tubes. The change in temperature was also determined accordingly on said days.
Blood sampling
Two millimeter of blood sample was collected from the cephalic veins of the dogs into a non-anticoagulant tube for evaluation of creatine kinase, lactate dehydrogenase and acetyl cholinesterase enzymes. The blood was allowed to clot in the laboratory and the serum separated into clean tubes. The tubes were stored in the freezer at -20°C until use. The Assessment of Biomarkers: Creatine kinase, Lactate dehydrogenase and Acetylcholinestase in the Experimental Groups. The blood level of creatine kinase and lactate dehydrogenase were determined using an Enzopak- CK-MB kit and LDH (MONOTM) kit respectively as described by the Reckon Diagnostics P. LTD an Iso 13485:2003 and GMP certified company. The blood level of acetylcholinesterase was determined using a technique as described by Ref. [13].
Assay procedure:
1. A 0.4 mL of the test serum was added to a cuvette containing 2.6 mL of phosphate buffer (0.1 m, pH 8.0) and 100 μL of DTNB.
2. The contents of the cuvette were thoroughly mixed by bubbling air and the absorbance was measured at 412 nm in an LKB spectrophotometer. The stable value on the spectrophotometer was recorded as the base line reading.
3. A 20 μL substrate of acetylthiocholine was added and the change in absorbance was recorded for a period of 20 minutes at intervals of 10 minutes. The change in the absorbance per minute was thus determined.
Statistical analysis
The data obtained was subjected to independent samples T test. The level of significance was accepted at probability less than p<0.05 (Kolmogorov Smirnov Test).
Results
From Figure 1, there was no significant difference (p<0.05) in the level of creatine kinase between the infected (GPA) and control group (GPB) by day 0 to 11 post infection. But by day 12 post infection there was a significant increase (p<0.05) in the T. brucei infected group (GPA) up till day 16 post infection compared to the control. In Figure 2 above, there was no significant difference (p<0.05) in the level of LDH between T. brucei infected group and control up till day 12 post infection. Thereafter the mean LDH level became significantly higher (p<0.05) in (GPA) up till day 16 compared with the control (GPB). In Figure 3, there was no significant difference (p<0.05) in the level of ACTH between the infected group (GPA) and the control (GPB) starting from day 0 to 16 post infection.
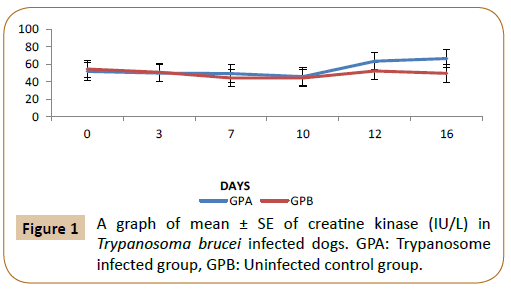
Figure 1: A graph of mean ± SE of creatine kinase (IU/L) in Trypanosoma brucei infected dogs. GPA: Trypanosome infected group, GPB: Uninfected control group.
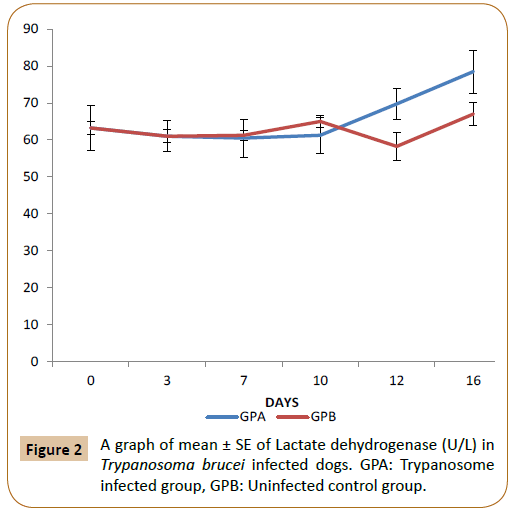
Figure 2: A graph of mean ± SE of Lactate dehydrogenase (U/L) in Trypanosoma brucei infected dogs. GPA: Trypanosome infected group, GPB: Uninfected control group.
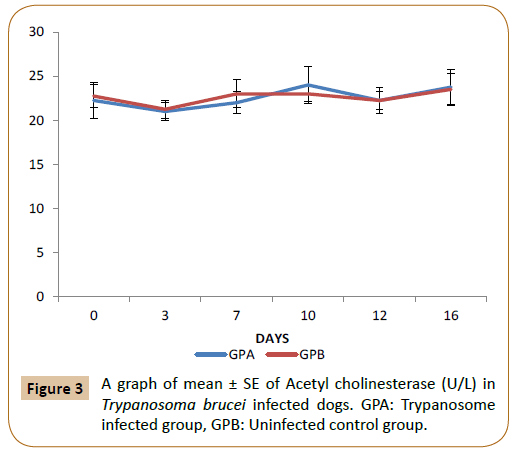
Figure 3: A graph of mean ± SE of Acetyl cholinesterase (U/L) in Trypanosoma brucei infected dogs. GPA: Trypanosome infected group, GPB: Uninfected control group.
In Table 1 below, there was no significant difference (p<0.05) in the temperature between T. brucei infected group and control till day 3 post infection. By day 7, there was a significant increase (p<0.05) which continued till day 10. It however showed no significant difference on day 12 post infection but by day 16, there was a significant rise (p<0.05) in temperature of T. brucei infected group (GPA) compared to the control (GPB).
| Experimental Period (Days) |
GPA |
GPB |
| 0 |
38.55 ± 0.24a |
38.13 ±0.34a |
| 3 |
38.78 ± 1.20a |
38.47± 1.00a |
| 7 |
39.58 ± 0.12a |
38.15 ±0.31b |
| 10 |
39.53 ± 0.19a |
38.13 ±0.27b |
| 12 |
39.10 ± 0.30a |
38.35 ± 0.50a |
| 16 |
39.05± 0.24a |
37.90 ± 0.14b |
Table 1: Mean ± SE of Temperature (°C) difference in Trypanosoma brucei infected dogs.Different superscripts (a,b) in a row indicate significant difference between the group means (p<0.05).
Discussion
Trypanosomosis is a very important disease of animals’ cattle, horses and canines in parts of the world where the disease is endemic. Despite the existence of some diagnostic techniques such as wet mount and hematocrit buffy coat techniques as described by Woo [12] which are to large extent reliable and sensitive in the diagnosis of trypanosomosis, there is often greater challenge in the diagnosis of animals infected by cases of tissue invasive Trypanosoma brucei. In such case the parasite sequestrates in tissues and organs where they cause varying degree of damage [14-16]. Such tissue parasites are often not detected using ordinary wet mount and haematocrit buffy coat technique and as such pose a challenge in early and effective diagnosis of tissue trypanosomosis in humans suffering from sleeping sickness and in animals particularly cattle suffering from chronic trypanosomosis. This results in undue delays in the institution of effective treatment of patients [17]. The usefulness of creatine kinase as a biomarker in medicine is observed in conditions that extensively affects the striated muscles, heart tissue and the brain. Trypanosoma brucei elicited an inflammatory disease condition in infected dogs with the release of diverse proteins including CK and LDH. Essentially, the trend in their alteration could be a pointer to a specific disease condition. For instance, in humans, a persistent decrease in creatine kinase activity is regarded as a biomarker of Huntington’s disease. This finding is regarded as a major landmark in the diagnosis, and monitoring of the treatment progress in Huntington’s disease [18]. Elevation in CK is essentially important in the diagnosis of myocardial infarction in humans [19]. Increase in creatine kinase was attributed to alteration in energy homeostasis in the body. Earlier, trypanosomosis has been associated with rapid consumption of energy in infected hosts [20,21]. And as such would induce a release of CK in response to energy deficit [22]. In addition, it could also be due to the pathophysiological effect of inflammation on muscle cells which caused infiltration of inflammatory cells. These cells induced lysis and release of intracellular proteins such as CK and myoglobin both harmful to the kidney [23]. Increase levels of CK and myoglobin maybe contributory to extensive kidney damage observed in Trypanosoma brucei infection in dogs [24].
Similarly, a significant increase (p<0.05) in lactate dehydrogenase was recorded in the study. Although LDH is abundant in tissue cells, its blood levels are usually maintained at low concentrations except in cases of tissue damage by injury or disease condition and as such is a useful tool in the diagnosis of tissue damage. Trypanosomosis has also been associated with varying degree of tissue damage [25]. And as earlier described the trend in alteration in the level of LDH could serve as biomarker of a disease condition. Earlier in medicine, a normal LDH-1/LDH-2 ratio of LDH co-enzymes is generally regarded as a reliable tool in confirming absence of heart attack and the level could also be assayed regularly to determine progress of patients under chemotherapy.
There may be limitations in the use of both CK and LDH as specific markers in Trypanosoma brucei infection in dogs despite significant increases in their levels. This is because similar increases could also be found in other inflammatory disease conditions however they would serve as surrogate markers of Trypanosoma brucei infection in dogs. This has been major limitation in the use of several biomarkers earlier detected in trypanosomosis in humans and animals [26,27]. However, the discovery of surrogate biomarkers of human trypanosomosis was highly desirable [28-30].
On the contrary, there was no significant difference (p<0.05) in the level of ACTH between the infected group (GPA) and the control. It therefore conflicts with the record of ACTH as biomarker of T. evansi infection in rabbits [31]. The confounding factors in both studies could be differences in species of animal and trypanosome used in the study. The significant increase (p<0.05) in the temperature of the infected group (GPA) was attributed to increase in parasitaemia [32,33]. The sudden disappearance of pyrexia on day 12 post infection was due to effect of circulating antibody against Trypanosoma brucei resulting in successive waves of parasitaemia a condition most characteristics of pathogenesis of Trypanosoma brucei infection. In conclusion, a significant increase (p<0.05) in the levels of CK and LDH would be used as surrogate markers of Trypanosoma brucei infection in dogs and thus could aid in the diagnosis and monitoring progress in treatment.
References
- Food and Agriculture Organization of the United Nations (2010) Tsetse and Trypanosomosis information. Programme against African Trypanosomosis. Rome, Volume 33, Part 1.
- Morrison WI, Murray M, Sayer PD, Preston JM (1981) The pathogenesis of experimentally induced Trypanosoma brucei infection in the dog. I. Tissue and organ damage. Am J Pathol 102: 168-181.
- Onyeyili RA, Egwu GO (1995) Chemotherapy of African Trypanosomosis: A historican review. Protozoology Abstract 5: 229-243.
- Boa FY (1997) The causes of failure of Human African trypanosomiasis control and the strategies for the future. OAU/STRC. Third meeting of STRCl, p: 348.
- Diack A, Maloo AK, Peregrine AS (1998) Effects of multiple treatments of cattie with draminazene on infectivity of drug resistance to T. congolense for Glossina morsitans centralis. Rev Elev Med Vet Pays Trop 51: 211-218.
- Brower V (2011) Biomarkers: Portents of malignancy. Nature 471: S19-S21.
- Thomson RA, Vinhaes MC, Rodríguez M, Monroy J, Persaud N, et al. (2005) International Meeting on Surveillance and Prevention of the Chagas Disease in the Amazônia: implementation of the Inter-government Initiative Surveillance and Prevention of the Chagas Disease in the Amazônia. Manaus, State of Amazon, Brazil, 19-22 of September of 2004. Rev Soc Bras Med Trop 38: 82-89.
- Craig-Schapiro R, Fagan AM, Holtzman DM (2009) Biomarkers of Alzheimer's disease. Neurobiol Dis 35: 128-140.
- Loukopoulo P, Mungall BA, Straw RC, Thornton JR, Robinson WF (2003) Matrix metalloproteinase-2 and -9 involvement in canine tumors. Vet Pathol 40: 382-394.
- Loukopoulos P, Thornton JR, Robinson WF (2003) Clinical and pathologic relevance of p53 index in canine osseous tumors. Vet Pathol 40: 237-248.
- Herbert WJ, Lumsden WHR (1976) Trypanosoma brucei: A rapid matching method for estimating the host’s parasitaemia. Experimental Parasitology 40: 427-428.
- Woo PTK (1970) The Haematocrit centrifugation technique for the diagnosis of African trypanosomosis. Acta Trop 27: 384-386.
- Ellman GL, Courtney KD, Andres V Jr, Feather-stone RM (1961) A new and rapid colorimeter determination of acetylcholinesterase activity. Biochem Pharmacol 7: 88-95.
- Akpa PO, Ezeokonkwo RC, Eze CA, Anene BM (2008) Comparative efficacy assessment of pentamidine isethionate and diminazene aceturate in the chemotherapy of Trypanosoma brucei brucei infection in dogs. Vet Parasitol 151: 139-149.
- Ekanem JT, Yusuf OK (2008) Some biochemical and haematological effects of black seed (Nigella sativa) oil on T. brucei infected rats. African J Biomed Res 11: 79-85.
- Akanji MA, Adeyemi OS, Oguntoye SO, Sulyman F (2009) Psidium guava extract reduces trypanososis associated lipid peroxidation and raises glutathione concentration in infected animals. Excli Journal 8: 148-154.
- Maloba F, Kagira J, Gitau G (2011) Astrocytosis as a biomarker for late stage human African trypanosomiasis in the vervet monkey model. Scientia Parasitologica 12: 53-59.
- Kim J, Moody JP, Edgerly CK, Bordiuk OL, Cormier K, et al. (2010) Mitochondrial loss, dysfunction and altered dynamics in Huntington’s disease. Hum Mol Genet 19: 3919-3935.
- Burtis CA, Ashwood ER (1999) Textbook of Clinical Chemistry. Published by WB Saunders Company, Philadelphia, USA.
- Eloy LJ, Lucheis SB (2009) Canine trypanosomiasis: etiology of infection and implications for public health. J Venom Anim Toxins Incl Trop Dis 15: 1-23.
- Takeet MI, Fagbemi BO (2009) Haematological, pathological and plasma Biochemical changes in rabbits experimentally infected with Trypanosoma congolense. Sci World J 4: 1-8.
- Ellington WR, Suzuki T (2007) Early evolution of the creatine kinase gene family and the capacity for creatine biosynthesis and membrane transport. Subcell Biochem 46: 17-26.
- Mehdi M, Negar M, Jeffrey B (2014) Increased Serum Creatine Kinase. Clinc Chem 60: 1-2.
- Nwoha RIO, Eze IO, Anene BM (2013) Serum biochemical and liver enzymes changes in dogs with single and conjunct experimental infections of Trypanosoma brucei and Ancylostoma caninum. African J Biotech 12: 618-624.
- Akanji MA, Adeyemi OS, Oguntoye SO, Sulyman F (2009) Psidium guava extract reduces trypanososis associated lipid peroxidation and raises glutathione concentration in infected animals. Excli Journal 8: 148-154.
- Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, et al. (2011) Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. Int J Cardiol 151: 18-33.
- Bautista-López NL, Morillo CA, López-Jaramillo P (2013) Matrix metalloproteinases 2 and 9 as diagnostic markers in the progression to Chagas cardiomyopathy. Am Heart J 165: 558-566.
- Kennedy PG (2008) The continuing problem of human African trypanosomiasis (sleeping sickness). Ann Neurology 64:116-126.
- Kennedy PG (2008) Diagnosing central nervous system trypanosomiasis: two stage or not to stage. Trans R Soc Trop Med Hyg 102: 306-307.
- Amin DN, Ngoyi DM, Nhkwachi GM, Palomba M, Rottenberg M, et al. (2010) Identification of stage biomarkers for human African trypanosomiasis. Am J Trop Med Hyg 82: 983-990.
- Márcio MC, Aleksandro SS, Francine CP, Raqueli F, Guilherme LD (2012) Cholinesterase as inflammatory markers in a experimental infection by Trypanosoma evansi in rabbits. An Acad Bras Cienc 84: 1105-1113.
- Ezeokonkwo RC, Ezeh IO, Onunkwo JI, Obi PO, Onyenwe IW, et al. (2010) Comparative haematological study of single and mixed infections of mongrel dogs with Trypanosoma congolense and Trypanosoma brucei brucei. Veterinary Parasitology 173: 48-54.
- Nwoha RIO, Anene BM (2011) Clinical signs and pathological changes in dogs with single and conjunct experimental infections of Trypanosoma brucei brucei and Ancylostoma caninum. Journal of Veterinary Parasitology 25: 97-102.