Keywords
Sensorineural hearing loss, Serum creatinine, diabetes Mellitus.
Introduction
Alterations in inner ear glucose concentration occur in diabetes and may alter hearing. Changes in systemic insulin concentration can alter the endolymphatic potential by altering the glucose concentration [1, 2]. Although many reports in the literature have evaluated the relationship between SNHL and DM, but there is no large-scale study of both laboratory and audiometric data in diabetic and SNHL available in our country.
Hence this study was attempted to identify whether diabetics have higher incidence of sensorineural hearing loss than general population and examine the duration of diabetes and its control on severity of hearing loss.
Materials and Methods
In a one year prospective study, 102 Type II diabetic patients and 118 nondiabetic rural population of age sex matched patients from the inpatient and outpatient of ENT and Medicine departments in our institution were tested on a audiometric measures, including pure-tone thresholds.
Fasting Blood sugar, post prandial Blood sugar, Serum creatinine, total cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, ECG, urine for micro and macro albuminuria, hemoglobin A1C levels and clinical examination of Fundus in all patients of both Diabetic and non diabetics were recorded.
Patients who are diagnosed of any psychiatric illnesses, substance abuse, or human immunodeficiency virus infection were eliminated from the study. Pure tone average were calculated from four frequencies (500 Hz, 1,000 Hz, 2,000 Hz, and 4,000 Hz) from each group of these patients. Patients were followed for 6 months and again pure tone average in four frequencies was calculated. Statistical analysis was done by karl Pearson’s chi square test to find the association between diabetes and hearing loss.
Result
In 102 diabetic and 118 non diabetic patients there were 68 male and 34 female in 102 diabetic patients and 80 male and 38 female in 118 Non diabetic patients.

Table 1: Number of male: female in both diabetic and non diabetic patient
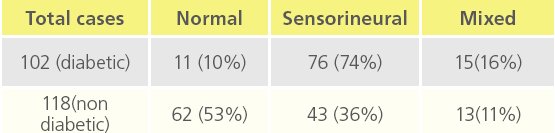
Table 2: “Deafness in diabetic and non diabetic patients”
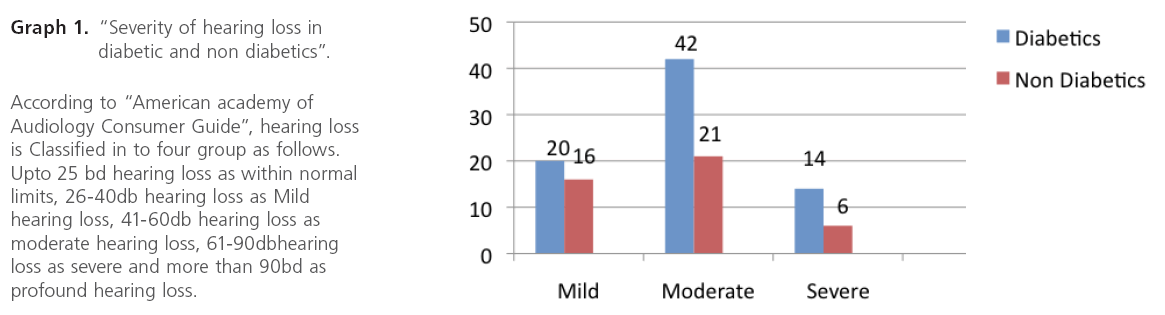
Graph 1: “Severity of hearing loss in diabetic and non diabetics”.
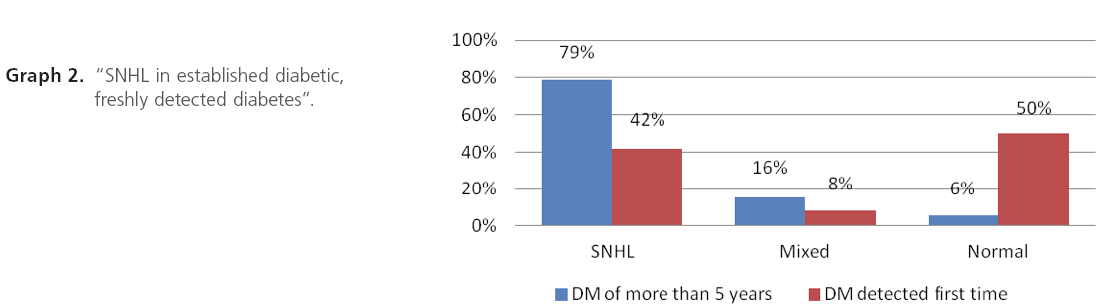
Graph 2: “SNHL in established diabetic, freshly detected diabetes”.
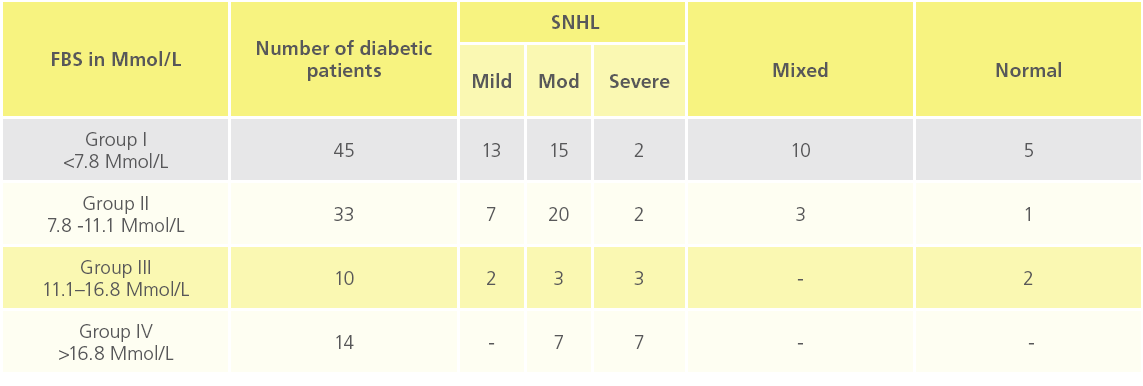
Table 3: “Distribution of deafness and its severity of SNHL at different blood sugar level”
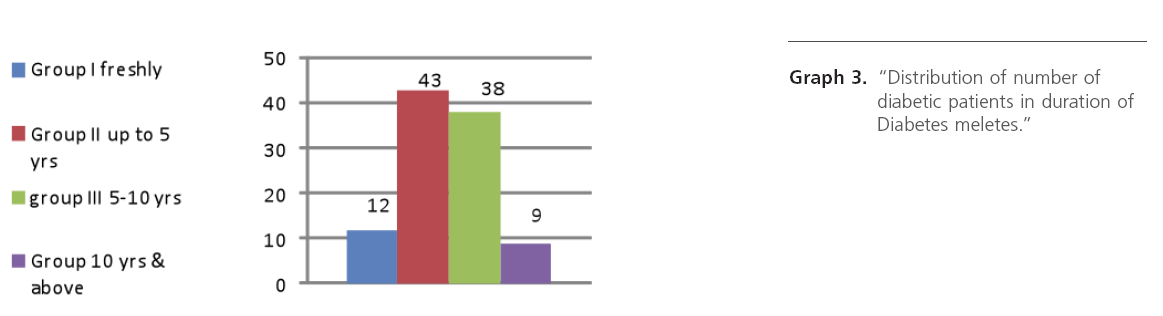
Graph 3: “Distribution of number of diabetic patients in duration of Diabetes meletes.”
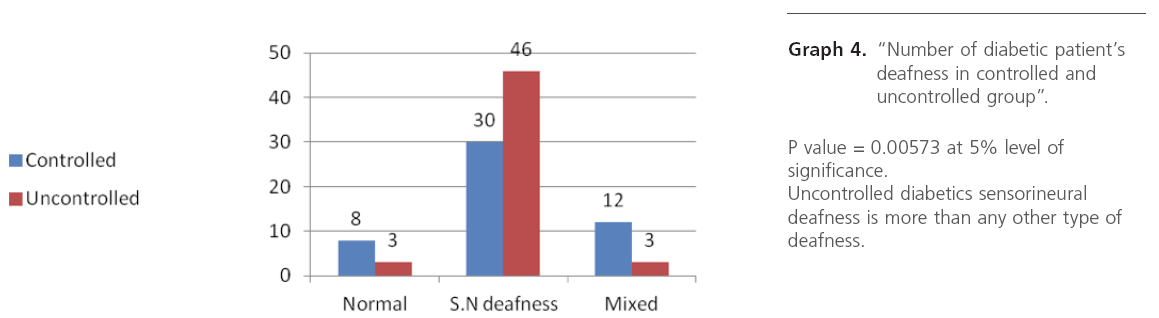
Graph 4: “Number of diabetic patient’s deafness in controlled and uncontrolled group”.
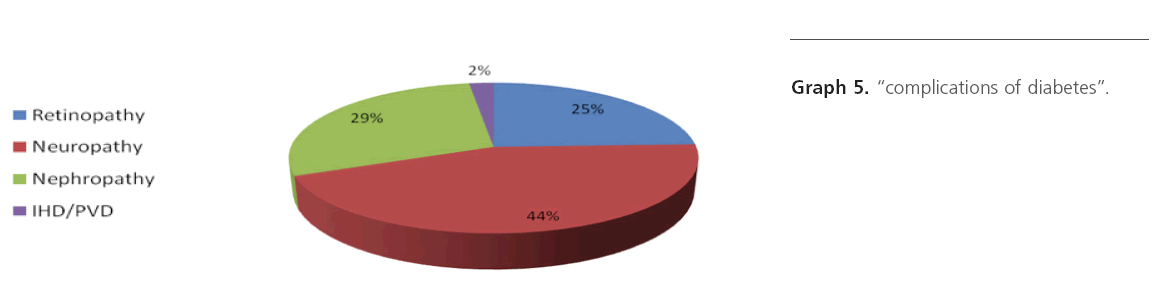
Graph 5: “complications of diabetes”.
In 102 diabetic patients, total number of cases affected with chronic complications were 69 and 33 cases were without any complication. Out of 69, neuropathy was seen in 32 patients and in remaining 37 patients had both retinopathy and nephropathy.
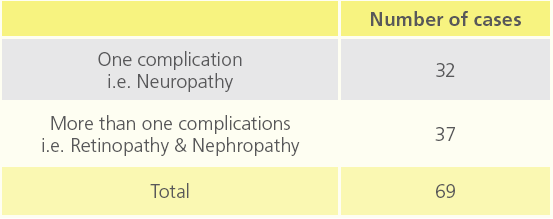
Table 4: Number of diabetic patients relating to co morbidity of diabetes.

Table 5a: “Deafness at different bone conduction threshold”
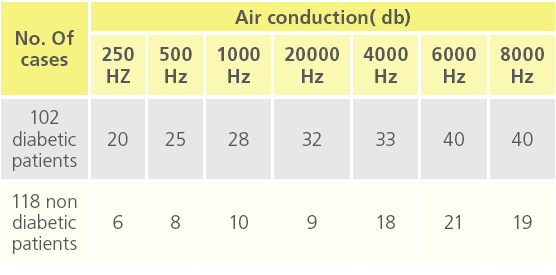
Table 5b: “deafness at different air conduction threshold”.
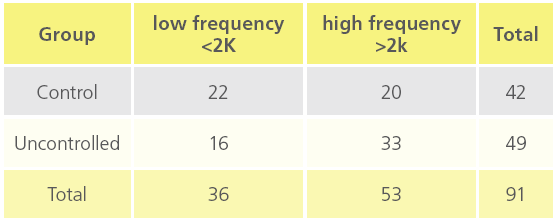
Table 5c: “Deafness at different BC threshold in controlled and uncontrolled diabetic patients”.

Table 5d: “Deafness at different AC threshold in controlled and uncontrolled diabetic threshold”
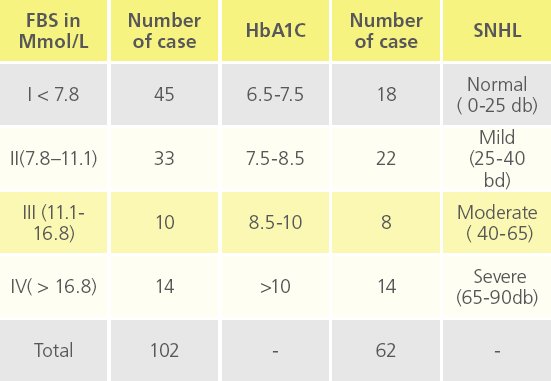
Table 5e: Deafness at different FBS levels with respect to HbA1C.
In 102 diabetic patients, 62 were followed up for 6 months and then HbA1c was recorded, whereas remaining 40 were lost during follow up at random.
HbA1C value 6.5-7.5 = 18 patients 7.5-8.5 = 22 patients 8.5-10 = 8 patients >10 = 14 patients Sensory neural deafness was noted
Sensory neural deafness was noted as mild, moderate and severe with respect to HbA1C levels.
Discussion
It is evident from a review of the otolaryngology literature that the relationship between diabetes and SNHL is complex. Most authors have concluded that patients with diabetes have worse hearing; however, a few authors, for example Harner [5] and Shuknecht[6], have denied a relationship between the two. Axelsson et al. [7] stated, “When the hearing in patients with diabetes is analyzed with respect to age group means, the results are essentially normal.” However, they did admit that some patients had a few abnormal findings, such as high-frequency pure tone loss, impaired speech discrimination ability, and abnormally decreased stapedial reflex thresholds. There is approximately a 5- to 30-dB difference in hearing thresholds across all frequencies, but more pronounced at 4 to 8 kHz [3,4,8]. It is important that most studies, including those of Celik et al. [4] and Cullen and Cinnamond 3, included patients with insulin-dependent diabetes mellitus (IDDM) only, and Huang et al.[8] included patients with insulin-dependent diabetes mellitus as well as patients with non–insulin-dependent diabetes mellitus (NIDDM).
Speech discrimination scores were generally seen not to be significantly different in diabetic patients, as shown by Cullen and Cinnamond[3]. However, Huang et al [8]observed a 7% worse discrimination score in diabetic patients. Snashall[9] observed lowered stapedial reflex thresholds in a small subgroup of seven patients, indicating recruitment and cochlear loss. This same group observed tone decay only at high frequencies.
According to Cullen and Cinnamond, male patients with diabetes had worse hearing than female patients with diabetes. They surmised that this may have been due to occupational noise exposure. However, Taylor and Irwin[10] observed that female patients with diabetes had significantly greater hearing loss than male patients with diabetes. Most studies in the literature reported no differences between the sexes. Age is another variable that could play a role in hearing loss. Axelsson et al[7] showed that the incidence of pure tone hearing loss increased with age in patients with diabetes, even after correction for presbycusis. Both processes involve progressive high-frequency losses. Potentially, the effects of diabetes could act synergistically with the processes underlying presbycusis to increase hearing loss.The duration of diabetes in relationship to hearing loss has also been investigated, with no clear conclusion. Celik et al.[4] observed that as the duration of diabetes increased to 15 years, the incidence of hearing loss increased. After 15 years of diabetes, the influence on hearing loss was not significant. However, Axelsson et al. [7] observed that age-matched patients with diabetes treated with insulin had better hearing that those patients treated with oral medications. In addition, the duration of diabetes of those patients treated with insulin was ten years longer than those treated orally. Wackym and Linthicum [11,12] observed that diabetic patients treated with diet alone had more severe hearing loss than those taking oral hypoglycemic agents, who had worse hearing than the patients taking insulin. This concept lends itself to the belief that aggressive therapy of diabetes leads to less hearing loss.
Attributing hearing loss to diabetes alone is often difficult because of other vascular diseases in these patients and because of compounding variables such as presbycusis. Duck et al 13 studied the interaction of hypertension in patients with IDDM and hearing loss. They observed that hypertension and diabetes had a synergistic effect on high-frequency SNHL. The micro vascular effects of hypertension are similar to those of diabetes, making these data plausible. This concept is important because the prevalence of hypertension in patients with diabetes varies between 10% and 80%, according to different reports. Intuitively, diabetic patients with retinopathy should have more hearing loss, given the similarity of the microvascular blood supply of the ear to that of the eye. Jorgensen[14] supports this, reporting hearing loss to be twice as common in diabetic patients with proliferative retinopathy.
The pathogenic effects of diabetes on the ear can be broadly grouped into neuropathic, angiopathic, and a combination of the two. The tissue effects of diabetes are thought to be related to the polyol pathway, where glucose is reduced to sorbitol. Sorbitol accumulation is implicated in neuropathy by causing a decrease in myoinositol content, abnormal phosphoinositide metabolism, and a decrease in Na+/ K+ ATPase activity [15]. Makishima and Tanaka [16] observed severe atrophy of the spiral ganglion in the basal and middle turns of the cochlea in diabetic patients with SNHL. They also observed that the VIIIth nerve showed signs of myelin degeneration, with fibrosis of the perineurium. This group also observed that atherosclerosis, a well-documented con sequence of diabetes and hypertension, was responsible for neuronal degeneration in the inner ear. Jorgensen [14] was the first to study the histopathologic properties of temporal bones in patients with diabetes with hearing loss, and observed thickening of the walls of the vasa nervorum of the VIIIth nerve, leading to acoustic neuropathy. Jorgensen also noted microangiopathic changes in the stria vasculariz. Wackym and Linthicum [11] observed microangiopathic changes in the endolymphatic sac, stria vasculariz, and basilar membrane. The diabetic patients with microangiopathic changes in the endolymphatic sac were noted to have the greatest degree of hearing loss.Genetic factors may also play a role in SNHL in a small subset of the population with diabetes and hearing loss. Van den Ouweland et al [17] observed a mutation in mitochondrial tRNA in a small subset of patients with maternally inherited diabetes with SNHL. Kadowaki et al [18] observed a similar mutation in mitochondrial DNA in the same type of patients. Since then, a variety of mitochondrial disorders that cause hearing loss have been identified; however, these seem to be uncommon. Recently, a small study demonstrated abnormalities of outer hair cell function and abnormal auditory brainstem responses in patients with diabetes.[19]
Using a rat model of diabetes, investigators have studied the histological effect of hyperglycemia on the inner ear. Smith et al 20 observed that the basement membranes of IDDM-induced rats (by streptozotocin injection, which causes pancreatic destruction) were nearly twice as thick as those of control rats. McQueen et al. [21] performed a similar experiment in a strain of rats with NIDDM (SHR/N-cp) and observed that NIDDM alone did not cause statistically significant basement membrane thickening.
Because many of the previous studies give conflicting evidence and their samples were small, we used a data mining approach to examine a large pool of patients. The VA started maintaining electronic medical records in 1989. This allows the examination and correlation of large pools of data over long periods of time. It is well documented that diabetes is a major cause of chronic renal failure by causing glomerular basement membrane thickening as well as hyalinization of afferent and efferent arterioles, leading to glomerulosclerosis and proteinaceous depositions in the kidney [15]. Diabetes also causes accelerated atherosclerosis by glycosylation of various lipoproteins. [15] Despite this, creatinine, total cholesterol, LDL cholesterol, and triglyceride levels were not statistically different between the diabetic populations with or without SNHL. Although the average creatinine level was higher in patients with diabetes than in those without diabetes (3.78 mg/dL vs. 1.29 mg/dL), total cholesterol and LDL levels were slightly lower in the diabetic population than in the nondiabetic population (195.44 mg/dL and 117.93 mg/dL, compared with 195.55 mg/dL and 122.13 mg/dL). However, the triglyceride levels were higher in the diabetic group (192.57 mg/dL vs. 156.99 mg/dL). This perhaps is because patients with diabetes are more aggressively treated with antilipemic medications that lower LDL and total cholesterol but have a lesser effect on triglyceride levels.
We observed that Out of 102 diabetic cases , 76 cases had SNHL and 15 patients had mixed hearing loss and deafness observed was mainly moderate type and male : female ratio was 2;1 and incidence of hearing impairment was also slightly more than in males as compared to females.
Observations of blood glucose level and deafness shows, maximum incidence of moderate type of sensorineural deafness in high glucose level, while compared with normal range blood glucose level.
While studying duration of diabetes and deafness, it was observed that, as duration of diabetes increases there is maximum incidence of sensorineural deafness and it was moderate type SNHL
Metabolic control of diabetes was studied, and we observed maximum incidence of moderate SNHL in very poor metabolic control or uncontrolled diabetes, from this study we conclude that there is relation between diabetic control and hearing threshold ie uncontrolled diabetics have higher hearing threshold than controlled. Sensory neural deafness was noted as mild, moderate and severe with respect to HbA1C levels.
While studying we came across many diabetic complications, cases have been observed with one or more than one complications. Few cases were observed without complication. We observed more incidence of moderate to severe type of SNHL in cases with diabetic complications. It suggests that there is relation between hearing threshold and complications of diabetes, i.e. diabetics with complications have higher hearing threshold.
Conclusion
There is a association between hearing threshold and diabetes mellitus. Hearing threshold in diabetics is increased and there is direct correlation between high blood glucose level and high hearing threshold.
Established diabetics are having more hearing impairment as compared to freshly detected diabetics probably due to long duration of diabetes and hearing threshold increases as the duration of diabetes increases.
Uncontrolled diabetics have higher hearing impairment than controlled and as a diabetic complications increases hearing impairment increases.
Though this study convincingly establishes the role of diabetes in hearing loss we suggest that auditory brainstem evoked response should be evaluated in the sample study to denote the probable site of involvement of inner ear and auditory pathways. This could not be done in our settings because of lack of facilities.
2495
References
- Jordao AMD (1857) Consideration sur un cas du diabete. Union Medicale du Paris; 11:446.
- Edgar TO.(1915) Klinische Untersuchungen uber die Erkrankungen des Gehororgans bei Diabetes Mellitus mit besonderer Berucksichtigung der Erkrankungen des inneren Ohres. Mschr Ohrenheilk LaryngoRhinol; 49:225–260.
- Harner SG (1981) Hearing in adult-onset diabetes mellitus. Otolaryngol Head Neck Surgery; 89:322–327.
- Celik O, Yalcin S, Celebi H, et al (1996) Hearing loss in insulindependent diabetes mellitus. Auris Nasus Larynx ; 23:127–132.
- Cullen JR, Cinnamond MJ (1993) Hearing loss in diabetics. J Laryngol Otol ; 107:179–182.
- Schuknecht HF(1993) Pathology of the Ear. 2d ed. Philadelphia: Lea & Febiger ; 312.
- Axelsson A, et al (1978) Hearing in diabetics. Acta Otolaryngol ; 356(Suppl):3–21. Diabetes and Sensineural hearing Loss 385 Otology & Neurotology, Vol. 24, No. 3, 2003
- Huang YM, Pan CY, Gu R, et al (1992) Hearing impairment in diabetics. Chin Med ; 105:44–48.
- Snashall SE.(1997). Bekesy audiometry and tone and reflex decay tests in diabetics. Arch Otolaryngol; 103:342–343.
- Taylor IG, Irwin J (1978) Some audiological aspects of diabetes mellitus. J Laryngol Otol; 92:99–113.
- Wackym PA, Linthicum FH Jr (1986) Diabetes mellitus and hearing loss: clinical and histopathologic relationships. Am J Otol; 7: 176–182.
- Schuknecht HF. (1993) Pathology of the Ear. 2nd ed. Philadelphia: Lea & Febiger; 416.
- Duck SW, Prazma J, Bennett PS, et al (1997) Interaction between hypertension and diabetes mellitus in the pathogenesis of sensorineural hearing loss. Laryngoscope; 107:1596–1604.
- Foster D. (1991) Harrison’s Principles of Internal Medicine. 12th ed. New York: McGraw-Hill; 1754–1755.
- Makishima K, Tanaka K(1971). Pathological changes of the inner ear and central auditory pathway in diabetics. Ann Otol Rhinol Laryngol; 80:218–228.
- Jorgensen MB. (1961) The inner ear in diabetes mellitus. Arch Otolaryngol; 74:373–381.
- Van den Ouweland JM, Lemkes HH, Tremblath RC, et al (1994) Maternally inherited diabetes and deafness is a distinct subtype of diabetes and associated with a single point mutation in the mitochrondrial tRNA (LeuUUR) gene. Diabetes; 43:746–751.
- Kadowaki T, Kadowacki H, Mori Y, et al (1994) Subtype of diabetes mellitus associated with a mutation of mitochondrial DNA. N Engl J Med; 330:962–968.
- Lisowska G, Namyslowski G, Morawski K, Strojek K(2001) Early identification of hearing impairment in patients with type 1 diabetes mellitus. Otol Neurotol ; 22:316–320.
- Smith TL, Raynor E, Prazma J, et al (1995) Insulin-dependent diabetic microangiopathy in the inner ear. Laryngoscope; 105:236–240.
- McQueen CT, Baxter A, Smith TL, et al (1999) Non-insulin-dependent diabetic microangiopathy in the inner ear. J Laryngol Otol ; 113: 13– 18.



















