 |
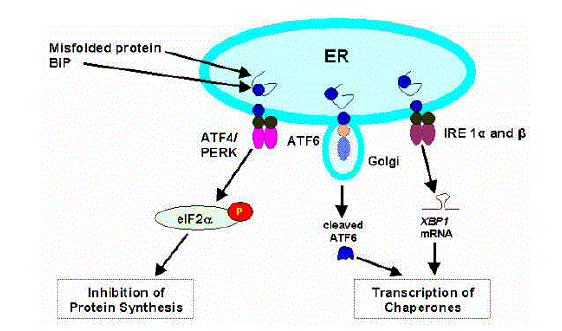
| Figure 4. The unfolded protein response (UPR) to endoplasmic reticulum (ER) stress. The UPR is a particularly important cellular response for cells that must accommodate high loads of secretory proteins like the beta cell. The UPR is principally mediated by ATF4/PERK, ATF6 and IRE which are activated by the abundant ER chaperone BiP. Misfolded proteins activate IRE1 and PERK by phosphorylation. IRE1 is a kinase that contains an ER regulatory domain and an RNaseL domain. IRE1 activation leads to upregulation of XBP1 which subsequently activates a family of genes encoding ER chaperones. PERK activation results in the phosphorylation of eIF2alpha that leads to a generalized inhibition of translation initiation. Finally, ATF6, a leucine-zipper transcription factor, transits into the Golgi following activation where it is cleaved into an active transcription factor for chaperones. |