Value of Salivary Gland Scintigraphy after Initial 131 I Therapy for Differentiated Thyroid Carcinoma: Prediction of Salivary Gland Dysfunction after High Cumulative Dose 131 I Administration
Yasuhiro Maruoka1*, Shingo Baba1, Takuro Isoda1, Yoshiyuki Kitamura1, Koichiro Abe2, Masayuki Sasaki3 and Hiroshi Honda1
1Department of Clinical Radiology, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
2Department of Diagnostic Imaging and Nuclear Medicine, Tokyo Women’s Medical University, Japan
3Department of Health Sciences, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan
- *Corresponding Author:
- Yasuhiro Maruoka, M.D
Department of Clinical Radiology
Graduate School of Medical Sciences
Kyushu University
3-1-1 Maidashi, Higashi-Ku Fukuoka-city
Fukuoka 812-8582, Japan
Tel: (81)-92-642-5695
E-mail: ymaruoka@radiol.med.kyushu-u.ac.jp
Received Date: October 17, 2017; Accepted Date: October 25, 2017; Published Date: November 10, 2017
Citation: Maruoka Y, Baba S, Isoda T, Kitamura Y, Abe K, et al. (2017) Value of Salivary Gland Scintigraphy after Initial 131 I Therapy for Differentiated Thyroid Carcinoma: Prediction of Salivary Gland Dysfunction after High Cumulative Dose 131I Administration. J Oncopathol Clin Res. 1:4.
Abstract
Objectives: Dry mouth symptoms induced by 131I therapy for differentiated thyroid carcinoma (DTC) can impair patients’ quality of life over the long term. We investigated the value of early SGS findings in predicting the development of dry mouth symptoms in patients receiving high cumulative dose 131I therapy for DTC.
Materials and method: Eighty DTC patients who underwent SGS using 370 MBq of 99mTc-pertechnetate after the initial and second rounds of 131I therapy (cumulative 131I dose >10 GBq) between January 2010 and December 2013 were retrospectively analyzed. SGS was assessed using a 3-point uptake score and washout rate (%WR) after lemon-juice stimulation in the parotid (PG) and submandibular glands (SMG). Summed scores from bilateral PG and SMG were defined as the summed uptake score (SUS) and summed WR score (SWRS), respectively. Risk factors for dry mouth symptoms were analyzed by uni- and multivariate logistic regression analyses.
Results: SUS/SWRS after initial 131I therapy were significantly associated with dry mouth symptoms both in univariate analysis (χ2 and odds ratio: 39.5 and 0.03 for SUS, and 46.4 and 0.02 for SWRS, respectively) and in multivariate analysis (χ2 and odds ratio: 6.13 and 0.06 for SUS, and 8.40 and 0.04 for SWRS, respectively).
Conclusion: SGS after the initial 131I therapy can be a feasible biomarker for predicting development of dry mouth symptoms secondary to high-dose 131I therapy.
Keywords
131I Therapy; Differentiated thyroid carcinoma; Dry mouth symptoms; Salivary gland dysfunction; Salivary gland scintigraphy
Introduction
Radioiodine therapy with 131I after total or near-total thyroidectomy is known to be an effective treatment for differentiated thyroid carcinoma (DTC) [1]. Several studies have reported a significant reduction in the rates of disease recurrence and cause-specific mortality in DTC patients after 131I therapy [2,3]. While 131I therapy is generally considered to be safe, a high cumulative dose has been reported to cause early- and late-onset complications in some cases [4,5]. Salivary gland damage is the most common long-term sideeffect after high-dose 131I therapy [4-12]. As salivary glands and thyroid tissue possess the same sodium−iodine symporter molecule [4,13,14], 131I accumulates and reaches a high concentration (about 30–40 times of that in the plasma) in the salivary glands [13]. β-radiation from the accumulated 131I causes local cytotoxic side-effects, manifesting as salivary gland damage and chronic sialadenitis. Chronic sialadenitis affects 11%–43% of patients treated with 131I therapy [4,15,16] and presents with dry mouth or dysphagia. These symptoms of salivary gland dysfunction significantly impair patients’ quality of life over the long term. Therefore, it is important that salivary gland dysfunction is quantitated objectively, and that patients at risk of development such symptoms are identified and treated early.
Previous studies have used salivary gland scintigraphy (SGS) for quantitative evaluation of parenchymal impairment of salivary glands in patients after 131I therapy for DTC. Bohuslavizki et al. have reported a progressive reduction in salivary gland function with the increase in the cumulative 131I dose (15% after 0.4–0.6 GBq, 30% after 1.4–1.5 GBq, and up to 90% after 24 GBq 131I) [17]. Caglar et al. [18] and Rasa et al. [19] have also reported a correlation between salivary gland dysfunction in SGS findings and the dose of 131I administered, as well as subjective symptoms, such as xerostomia. However, SGS results are generally subjectively interpreted and objective measures based on SGS findings that can be used for quantitative assessment of salivary gland dysfunction are lacking.
A quantitative SGS-based marker of salivary gland dysfunction could potentially be used as a predictive biomarker of dry mouth symptoms arising from high-dose 131I therapy. The purpose of this study was therefore to investigate the prediction of dry mouth symptoms after administration of a high cumulative 131I dose, using SGS findings after the initial 131I therapy for DTC.
Materials and Methods
Patients
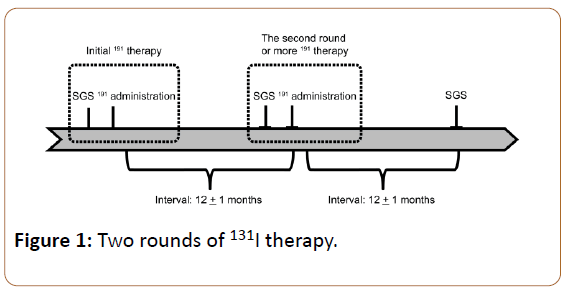
This study was approved by our institutional review board and written informed consent from each patient was obtained. Data of 80 patients with DTC (29 men and 51 women) who underwent at least two rounds of 131I therapy and had SGS after each round of 131I therapy, between January 2010 and December 2013, were retrospectively analyzed (Figure 1). The patients ranged from 16−72-years-old (median: 53 years), and were histopathologically diagnosed with either papillary or follicular carcinoma. The inclusion criteria were as follows: (i) patients who underwent 131I therapy for metastatic or recurrent DTC tumors; and (ii) patients who had a cumulative administered 131I dose exceeding 10 GBq. The exclusion criteria were as follows: (i) known history of salivary gland resection; (ii) known history of external radiation therapy in the neck; (iii) baseline disease with salivary gland dysfunction before the first 131I therapy (e.g., Sjogren's syndrome or salivary gland tumor); (iv) usage of anticholonergic drugs and other drugs causing xerostomia; and (v) suboptimal studies or technical errors in SGS (e.g., motion artifacts, inadequate injection technique).
All patients underwent thyroid hormone withdrawal for at least 3 weeks before 131I therapy. A low-iodine diet was started 2 weeks before 131I therapy. The 131I dose ranged from 3.7 to 5.5 GBq, as per treatment dose. SGS was performed 12 ± 2 months after the initial and second round of therapy. The patient characteristics are shown in Table 1.
| Age (years) | 16–72 (median: 53) |
| Men / women | 29 / 51 |
| Papillary / follicular | 68 / 12 |
| TG level before 131I therapy (ng/mL) | 7.9–54400 (median: 115) |
| Cumulative 131I administered dose (GBq) | 11.4–31.5 (median: 115) |
| TNM stage | |
| I | 11 (14%) |
| II | 16 (20%) |
| III | 3 (4%) |
| IVA | 17 (21%) |
| IVB | 0 (0%) |
| C | 33 (41%) |
| ATA risk classification | |
| Intermediate | 31 (39%) |
| High | 49 (61%) |
TG = Thyroglobulin; ATA = American Thyroid Association
Table 1: Baseline characteristics of the patients.
SGS
Imaging was performed using a hybrid camera combining a dual-head camera with spiral computed tomography (CT), using the same gantry (Infinia; GE Healthcare, Little Chalfont, UK) equipped with a low-energy parallel-hole collimator. The patient was placed in a supine position, and the camera was positioned for an anterior head-and-neck projection. Dynamic imaging was performed in a 64 × 64 pixel matrix, at 5 min per frame, starting immediately after a bolus intravenous injection of 370–444 MBq (10–12 mCi) 99mTc-pertechnetate. Imaging continued for 40 min after injection. At 23 min after injection, each patient was given lemon juice stimulation in the mouth, without moving, under continuous imaging.
Analysis of SGS findings
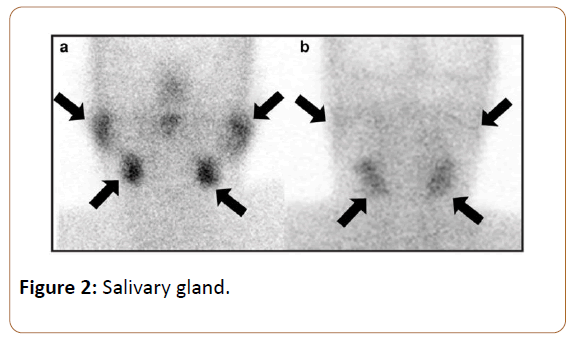
SGS images taken immediately before lemon juice stimulation were used to assess the capacity for saliva production by the salivary glands. A 3-point uptake score was used to visualize the degree of 99mTc-pertechnetate uptake in each salivary gland (Figure 2) 0—decreased to background levels; 1—decreased, but more than background levels; 2— normal according to a previous article [20]. Scores of 0, 1 and 2 were respectively defined as non-function, dysfunction, and no dysfunction in each gland. The summed uptake score (SUS) derived by addition of the values from the bilateral parotid glands (PGs) and submandibular glands (SMGs) was considered to represent the overall salivary production status.
Next, the capacity for saliva secretion was measured by the washout rate, as follows. Regions of interest were drawn on the dynamic images of the bilateral PGs and SMGs, and time −activity curves were generated. The response of salivary glands to lemon juice was noted on the time−activity curves as a sharp decline in the activity of the gland, with a subsequent slow buildup. The washout rate (%WR) was defined as follows [21]:
%WR = (cmax – post counts) / cmax × 100
where ‘cmax’ is the prestimulatory maximum count value and ‘post counts’ is the post-stimulatory minimum count value. Using this formula, the %WR in the PG (PG %WR) and the %WR in the SMG (SMG %WR) were respectively calculated. According to the literature [19], decreased %WR was defined as a decrease in %WR more than 10% compared with %WR before the initial therapy.
Both the %WR in PGs (PG %WR) and %WR in SMGs (SMG %WR) were calculated. Finally, based on a previous study, the assessment of %WR was performed with a 3-point WR score, based on the findings in patients before 131I therapy, as follows: 0: % decreased WR = 100%; 1: 10%: < decreased %WR < 100%; 2: %WR ≥ 10% [20]. %WR scores of 0, 1, 2 were respectively considered to indicate non-function, dysfunction, and no dysfunction in salivary glands. %WR score before the initial 131I therapy was defined as 2 in all patients, based on that DTC patients before 131I therapy in this study have no salivary gland dysfunction. The summed WR score (SWRS) derived by addition of the values of the bilateral PGs and bilateral SMGs was taken as representing the overall salivary secretion status. SUS, PG %WR, SMG %WR and SWRS between the positive-symptom and negative-symptom groups are presented in Table 2.
| Symptom-negative group (n = 49) | Symptom-positive group (n = 31) | |
|---|---|---|
| Age (years old) | 48 ± 14 | 53 ± 16 |
| Men / women | 26 / 23 | 3 / 28* |
| Histological type | ||
| Papillary / follicular | 42 / 7 | 26 / 5 |
| TG level before 131I therapy (ng/mL) | 2312 ± 9543 | 2644 ± 6823 |
| Per treatment 131I administered dose | 4.2 ± 0.5 | 4.3 ± 0.5 |
| Cumulative 131I administered dose (GBq) | 13.8 ± 5.8 | 14.2 ± 5.7 |
| TNM stage | ||
| I/ II / III / IVA / VB / VIC | 10 / 9 / 2 / 11 / 17 | 1 / 7 / 1 / 6 / 16 |
| Risk group | ||
| Intermediate / high | 23 / 26 | 23-Aug |
| 131I scintigraphy findings in salivary glands after the first 131I therapy | ||
| Positive / negative | Oct-39 | 15 / 16* |
| Salivary gland scintigraphy parameters | ||
| SUS before the first 131I therapy | 8 ± 0 | 8 ± 0 |
| PG %WR before the first 131I therapy (%) | 59 ± 8 | 60 ± 7 |
| SMG %WR before the first 131I therapy (%) | 55 ± 6 | 56 ± 5 |
| SWRS before the first 131I therapy | 8 ± 0 | 8 ± 0 |
| SUS after the first 131I therapy | 7.7 ± 0.7 | 6.0 ± 1.4* |
| PG %WR after the first 131I therapy (%) | 56 ± 16 | 21 ± 21* |
| SMG %WR after the first 131I therapy (%) | 52 ± 16 | 47 ± 11 |
| SWRS after the first 131I therapy | 7.5 ± 1.0 | 4.6 ± 1.7* |
| SUS after the second 131I therapy | 7.1 ± 1.2 | 2.8 ± 1.4* |
| PG %WR after the second 131I therapy (%) | 51 ± 22 | 5 ± 11* |
| SMG %WR after the second 131I therapy (%) | 52 ± 8 | 32 ± 14* |
| SWRS after the second 131I therapy | 6.8 ± 1.6 | 2.7 ± 1.7* |
| Δ SUS | 0.6 ± 0.9 | 3.1 ± 1.5* |
| Δ SWRS | 0.7 ± 1.2 | 1.9 ± 1.6* |
SUS = Summed Uptake Score; SWRS = Summed Washout Rate Score; PG = Parotid Gland; SMG = Submandibular Gland
* p<0.001 vs. symptom-negative group.
Table 2: The difference between symptom-negative group and symptom-positive group.
Finally, the differences in SUS and SWRS between the initial and second 131I therapy sessions were defined as ΔSUS and ΔSWRS, respectively. Based on the presence or absence of dry mouth symptoms at the follow-up (at 12 ± 1 months after the last 131I therapy), the 80 patients were categorized into a positive-symptom group (n = 31 patients) and a negative- symptom group (n = 49 patients). SUS/SWRS and ΔSUS/ΔSWRS were compared between the positive-symptom and negative-symptom groups (Table 2).
Post-Therapy 131I Scintigraphy
Post-therapy 131I scintigraphy imaging was performed 5–7 days after 131I administration andsingle-photon emission computed tomography;
Analysis of Post-Therapy 131I scintigraphy findings
131I scintigraphy images were used to visualize the degree of 131I accumulation in the salivary glands, as a marker of the potential radiation exposure in salivary gland tissue. 131I accumulation that exceeded background levels in at least either one of salivary gland was defined as positive, and 131I accumulation equal to background levels in all salivary glands was defined as negative.
Statistical analysis
All statistical calculations were performed with JMP® package (version 13.0.0; SAS Institute). Comparisons of SUS/ SWRS and ΔSUS/ΔSWRS between positive-symptom and negative-symptom groups were performed with the Mann −Whitney U test. Risk factors related to the development of dry mouth symptoms (salivary gland dysfunction) were analyzed by uni- and multivariate logistic regression analysis. Regression coefficients and odds ratios were calculated and 95% confidence intervals were determined. The predictive value of factors for dry mouth symptoms was analysed by receiver operating characteristic (ROC) curve analysis. Statistical significance for all tests was set at p<0.05.
Results
Comparison of SUS/SWRS and ΔSUS/ΔSWRS between the Positive- and Negative-Symptom Groups
In the overall study population, PG and SMG SUS were both (mean ± SD) 8 (0) before the initial 131I therapy, and respectively 7.0 (1.4) (range: 2−8) and 6.4 (1.9) (range: 2−8) after the initial 131I therapy, and 5.5 (2.5) (range: 0−8) and 5.2 (2.6) (range: 0−8) after the second round of 131I therapy. Similarly, PG and SMG %WR were 64 ± 8% (49–86%) and 57 ± 6% (46–73%), respectively, before the initial 131I therapy, 41 ± 25% (0–79%) and 50 ± 11% (20–74%) after the initial 131I therapy, and 30 ± 27% (0–74%) and 44 ± 15% (0−65%) after the second round of 131I therapy (Table 2). Overall, 35 of 80 (43.7%) patients demonstrated decreased SUS and 38 of 80 (47.5%) patients had decreased SWRS after the initial 131I therapy.
Additionally, 31 of 80 (38.8%) patients suffered dry mouth symptoms after the second round of 131I therapy. SUS and SWRS were significantly less in the symptom-positive group than in the symptom-negative group, after both the initial and the second 131I therapy sessions (SUS: p<0.0001, SWRS: p<0.0001, at both instances). ΔSUS/ΔSWRS was significantly higher in the symptom-positive group than in the symptom-negative group (ΔSUS: p<0.0001, ΔSWRS: p=0.0003).
Risk factor analysis for dry mouth symptoms after 131I Therapy
Univariate logistic analysis showed that sex, 131I accumulation in salivary glands, SUS, and SWRS were significant association with dry mouth symptoms. After multivariate logistic analysis, only SUS and SWRS continued to show significant association with dry mouth symptoms (χ2 and odds ratio: 6.13 and 0.06 for SUS, and 8.40 and 0.04 for SWRS, respectively; (Table 3).
| Characteristics | Univariate logistic analysis | Multivariate logistic analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| χ2 | Odds | P | χ2 | Odds ratio | 95% CI | P | ||
| ratio | ||||||||
| Age (years) | ≥ 54 vs. < 54 | 3.21 | 0.44 | 0.07 | 0.02 | 0.81 | 0.03 – 11.1 | 0.88 |
| Sex | Women vs. men | 17.3 | 0.09 | < 0.0001 | 0.98 | 0.34 | 0.04 – 2.96 | 0.32 |
| Histological type | Papillary vs. follicular | 0.05 | 0.87 | 0.82 | 1.83 | 0.12 | 0.004 – 2.51 | 0.18 |
| TNM stage | III / IV vs. I/ II | 1.46 | 0.55 | 0.23 | 1.64 | 0.15 | 0.004 – 2.52 | 0.2 |
| ATA risk group | High vs. intermediate | 3.67 | 0.39 | 0.06 | 0.003 | 0.93 | 0.07 – 12.3 | 0.95 |
| Recurrent disease | Positive vs. negative | 0.31 | 0.73 | 0.58 | 0.24 | 0.59 | 0.06 – 4.85 | 0.63 |
| TG level before therapy (ng/mL) | ≥ 19 vs. < 19 | 0.43 | 0.71 | 0.51 | 0.35 | 0.46 | 0.03 – 6.70 | 0.56 |
| Per treatment 131I dose (GBq) | ≥ 4.1 vs. < 4.1 | 0.52 | 0.71 | 0.47 | 0.25 | 0.59 | 0.06 – 4.72 | 0.62 |
| Cumulative 131I dose (GBq) | ≥ 15.6 vs. < 15.6 | 1.57 | 0.55 | 0.21 | 2.65 | 0.09 | 0.003 – 1.61 | 0.1 |
| 131I accumulation in salivary glands | Positive vs. negative | 6.84 | 0.27 | 0.009 | 1.65 | 0.28 | 0.03 – 1.92 | 0.2 |
| SGS findings after initial 131I therapy | ||||||||
| SUS | ≤ 7 vs. > 7 | 39.5 | 0.03 | < 0.0001 | 6.31 | 0.06 | 0.003 – 0.55 | 0.01 |
| SWRS | ≤ 6 vs. > 6 | 46.4 | 0.02 | < 0.0001 | 8.63 | 0.04 | 0.001 – 0.37 | 0.003 |
ATA: American Thyroid Association; TG = Thyroglobulin; SGS: Salivary Gland Scintigraphy; SUS: Summed Uptake Score; SWRS: Summed Washout Rate Score.
Table 3: Risk factor analysis for dry mouth symptoms.
Predictive ability of SUS/SWRS for dry mouth symptoms
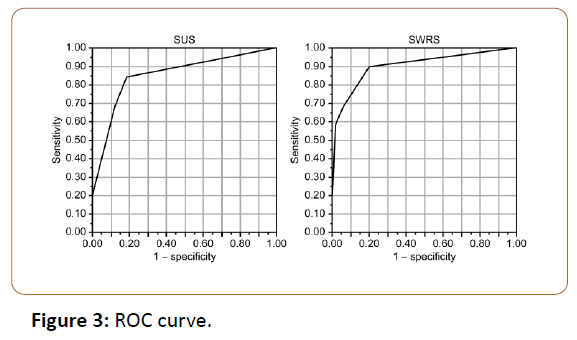
ROC curve analysis showed that symptom-positive and symptom-negative groups could be differentiated with 74% (23/31) sensitivity, 43% (21/49) specificity, 55% (44/80) accuracy, and an area under the curve (AUC) of 0.52, based on a cumulative 131I dose with a cutoff threshold of 15.6 GBq. Positive or negative findings of 131I accumulation in salivary glands differentiated symptom-positive and symptomnegative groups with 48% (15/31) sensitivity, 80% (39/49) specificity, 68% (54/80) accuracy. In comparison, SUS and SWRS, with a cutoff threshold of 7 and 6, differentiated between the two groups with 84% (26/31) and 90% (28/31) sensitivity, 82% (40/49) and 80% (39/49) specificity, 83% (66/80) and 84% (67/80) accuracy, and AUC of 0.85 and 0.90, respectively (Figure 3).
Discussion
We addressed the utility of SGS findings after the initial 131I therapy to assess the risk of dry mouth symptoms after high-dose 131I therapy in patients with DTC. While SUS and SWRS progressively declined in all patients after 131I therapy, the decline (i.e., ΔSUS and ΔSWRS) was significantly greater in the symptom-positive group than in the symptom-negative group in the present study. These results suggest that patients with significantly greater worsening of SUS/SWRS after the initial therapy developed salivary gland dysfunction during follow-up, after the second therapy. Our results therefore indicate that SGS findings after initial 131I therapy can potentially predict the risk of radiation-induced salivary gland damage after continued 131I therapy.
Furthermore, SUS and SWRS were shown to be independent risk factors for the development of dry mouth symptoms. While a cumulative 131I dose has previously been demonstrated to be a risk factor for salivary gland dysfunction after 131I therapy [2,10,16], we found that SUS and SWRS values had greater predictive ability for the development of dry mouth symptoms after 131I therapy than did the cumulative 131I dose. Symptomatic salivary dysfunction secondary to 131I therapy could result both from a decrease in saliva production and/or secretion into the mouth. In particular, SWRS, which is reflective of salivary secretion, showed high predictive ability for dry mouth symptoms. Mandel et al. have reported that increased capillary permeability in sialadenitis enhances 131I transmigration into the salivary parenchyma, while inflammation-induced duct wall damage and lumen obstruction promote 131I retention [22]. Similarly, Lee et al. have shown the importance of the clearance of saliva from salivary glands into the oral cavity as a risk factor for dry mouth symptoms due to salivary gland dysfunction secondary to 131I therapy [23].
On the other hand, our study did not find that 131I accumulation in salivary glands on post-therapy 131I scintigraphy was an independent risk factor for dry mouth symptoms. This is in contrast to a previous study that reported that symptomatic sialadenitis after radioiodine ablation could be predicted with high sensitivity by post-ablation 131I wholebody scintigraphy [23]. We speculate that 131I accumulation in salivary glands on post-therapy 131I scintigraphy mainly reflects the acute phase of sialadenitis secondary to 131I therapy. In contrast, SGS findings approximately 12 months after 131I therapy reflect a relatively chronic phase of sialadenitis, which may explain the superiority of SGS findings to post-therapeutic 131I scintigraphy findings in predicting dry mouth symptoms in our study.
Recently, sialoendoscopic intervention by dilating salivary ducts has been reported as treatment for chronic sialadenitis [24,25], and the intervention before salivary ducts is totally obstructed is favorable to obtain successful outcomes [24]. Early prediction of salivary gland dysfunction with dry mouth by SGS after initial 131I therapy may contribute to determining indication of sialoendoscopic intervention for chronic sialadenitis.
The present study has several limitations. First, the duration between the last 131I therapy and assessment of dry mouth symptoms in patients was approximately 1 year. Since salivary gland dysfunction is a late-onset complication that could develop even after 1 year, a longer follow-up period after 131I therapy may be required to assess the study outcomes adequately. Second, we could not include the amount of saliva in the mouth as an objective criterion of salivary gland dysfunction, due to the retrospective nature of our study.
In conclusion, we have shown that findings from SGS after an initial 131I therapy could be an effective tool for predicting the development of dry mouth symptoms secondary to highdose 131I therapy. We therefore recommend that SGS is performed in patients after initial 131I therapy, particularly when repeated and high-dose 131I therapy is anticipated.
References
- Tuttle RM, Leboeuf R, Shaha AR (2008) Medical management of thyroid cancer: a risk adapted approach. J Surg Oncol 97: 712–716.
- Mazzaferri EL, Jhiang SM (1995) Differentiated thyroid cancer long-term impact of initial therapy. Trans Am Clin Climatol Assoc 106: 151–170.
- Sawka AM, Brierley JD, Tsang RW, Thabane L, Rotstein L, et al. (2008) An updated systematic review and commentary examining the effectiveness of radioactive iodine remnant ablation in well-differentiated thyroid cancer. Endocrinol Metab Clin North Am 37: 457–480.
- Mandel SJ, Mandel L. Radioactive iodine and the salivary glands (2003) Thyroid 13: 265–271.
- Alexander C, Bader JB, Schaefer A, Finke C, Kirsch CM (1998) Intermediate and long-term side-effects of high-dose radioiodine therapy for thyroid carcinoma. J Nucl Med 39: 1551–1554.
- Ish-Shalom S, Durleshter L, Segal E, Nagler RM (2008) Sialochemical and oxidative analyses in radioactive I131-treated patients with thyroid carcinoma. Eur J Endocrinol 158: 677–681.
- Walter MA, Turtschi CP, Schindler C, Minnig P, Müller-Brand J, et al. (2007) The dental safety profile of high-dose radioiodine therapy for thyroid cancer: long-term results of a longitudinal cohort study. J Nucl Med 48: 1620–1625.
- Kita T, Yokoyama K, Higuchi T, Kinuya S, Takiet J, et al. (2004) Multifactorial analysis on the short-term side-effects occurring within 96 hours after radioiodine-131 therapy for differentiated thyroid carcinoma. Ann Nucl Med 18: 345–349.
- Allweiss P, Braunstein GD, Katz A, Waxman A (1984) Sialadenitis following I-131 therapy for thyroid carcinoma: concise communication. J Nucl Med 25: 755–758.
- Malpani BL, Samuel AM, Ray S (1996) Quantification of salivary gland function in thyroid cancer patients treated with radioiodine. Int J Radiat Oncol Biol Phys 35: 535–540.
- Hoelzer S, Steiner D, Bauer R, Reiners C, Farahati J, et al. (2000) Current practice of radioiodine treatment in the management of differentiated thyroid cancer in Germany. Eur J Nucl Med 27: 1465–1472.
- Solans R, Bosch JA, Galofré P, Porta F, Roselló J, et al. (2001) Salivary and lacrimal gland dysfunction (sicca syndrome) after radioiodine therapy. J Nucl Med 42: 738–743.
- De La Vieja A, Dohan O, Levy O, Carrasco N (2000) Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol Rev 80: 1083–1105.
- Shen DH, Kloos RT, Mazzaferri EL, Jhian SM (2001) Sodium iodide symporter in health and disease. Thyroid 11: 415–425.
- Silberstein EB (2008) Reducing the incidence of 131I-induced sialadenitis: the role of pilocarpine. J Nucl Med 49: 546–549.
- Grewal RK, Larson SM, Pentlow CE, Pentlow KS, Gonen M, et al. (2009) Salivary gland side-effects commonly develop several weeks after initial radioactive iodine ablation. J Nucl Med 50: 1605–1610.
- Bohuslavizki KH, Klutmann S, Jenicke L, Brenner W, Feyerabend B, et al. (1999) Radioprotection of salivary glands by S-2-(3-aminopropylamino)-ethylphosphorothioic (amifostine) obtained in a rabbit animal model. Int J Radiat Oncol Biol Phys 45: 181–186.
- Caglar M, Tuncel M, Alpar R (2002) Scintigraphic evaluation of salivary gland dysfunction in patients with thyroid cancer after radioiodine treatment. Clin Nucl Med 27: 767–771.
- Raza H, Khan AU, Hameed A, Khan, Ayub (2006) Quantitative evaluation of salivary gland dysfunction after radioiodine therapy using salivary gland scintigraphy. Nucl Med Commun 27: 495–499.
- Maruoka Y, Baba S, Isoda T, Kitamura Y, Abe K, et al. (2017) A Functional Scoring System Based on Salivary Gland Scintigraphy for Evaluating Salivary Gland Dysfunction Secondary to 131I therapy in Patients with Differentiated Thyroid Carcinoma. J Clin Diagn Res 11: TC23–28.
- Aung W, Murata Y, Ishida R, Takahashi Y, Okada N, et al. (2001) Study of quantitative oral radioactivity in salivary gland scintigraphy and determination of the clinical stage of Sjögren's syndrome. J Nucl Med 42: 38–43.
- Mandel SJ, Mandel L (2003) Radioactive iodine and the salivary glands. Thyroid 13: 265–271
- Lee SM, Lee JW, Kim SY, Han SW, Bae WK (2013) Prediction of risk for symptomatic sialadenitis by post-therapeutic dual 131I scintigraphy in patients with differentiated thyroid cancer. Ann Nucl Med 27: 700–709.
- Kim J, Han G, Lee S, Lee DY, Kim YM (2007) Sialoendoscopic treatment for radioiodine Induced sialadenitis. Laryngoscope 117: 133–136.
- Nahlieli O, Baruchin A. (2010) Long-term experience with endoscopic diagnosis and treatment of salivary gland inflammatory diseases. Laryngoscope 110: 988–993.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences